A team of King Abdullah University of Science and Technology (KAUST) researchers has innovated metal-free, ammonium-ion batteries that provide a sustainable alternative to power the green transition.
The batteries comprise ammonium-ion electrolytes that may provide an environmentally friendly competitor to traditional metal-ion batteries dependent on precious, finite, and consistently dwindling resources. The ammonium-ion batteries may also have uses in several fields, most notably for grid storage.
Challenges facing sustainable battery development
Metal-ion batteries, including lithium-ion batteries, are the most popular choice for energy storage currently due to their high energy density and versatility and are present in mobile phones, laptops, electric vehicles, and more.
Despite leading the battery sector, the metal ions utilised in their electrolytes come from limited and diminishing resources, which casts uncertainty over their long-term sustainability. Moreover, metal-ion batteries can be toxic and flammable, making them unsafe and harmful to the environment.
Although there have been various attempts to develop ammonium-ion-based batteries to solve these concerns due to ammonium cations being lightweight and easily synthesised and recycled. Despite this, ammonium cations are susceptible to reducing into hydrogen and ammonia at low operation potential, which inhibits the batteries’ performance. Additionally, they can rapidly dissolve in electrolytes, so they are, therefore, difficult to incorporate into electrode materials.
How KAUST advanced ammonium-ion design
The KAUST team designed a high-efficiency metal-free battery by amalgamating an ammonium-cation-containing electrolyte with carbon-based electrodes. This is beneficial because the graphite cathode and the organic semiconductor anode are cheap, environmentally friendly, and renewable.
For the ammonium cations, the researchers selected hexafluorophosphate ions as negative charge carriers and employed graphite’s ability to reversibly accommodate these anions within its layers, creating a ‘dual-ion’ battery. Within the battery, cations and anions simultaneously insert into their corresponding electrode throughout charge cycles and are emitted into the electrolyte when discharging.
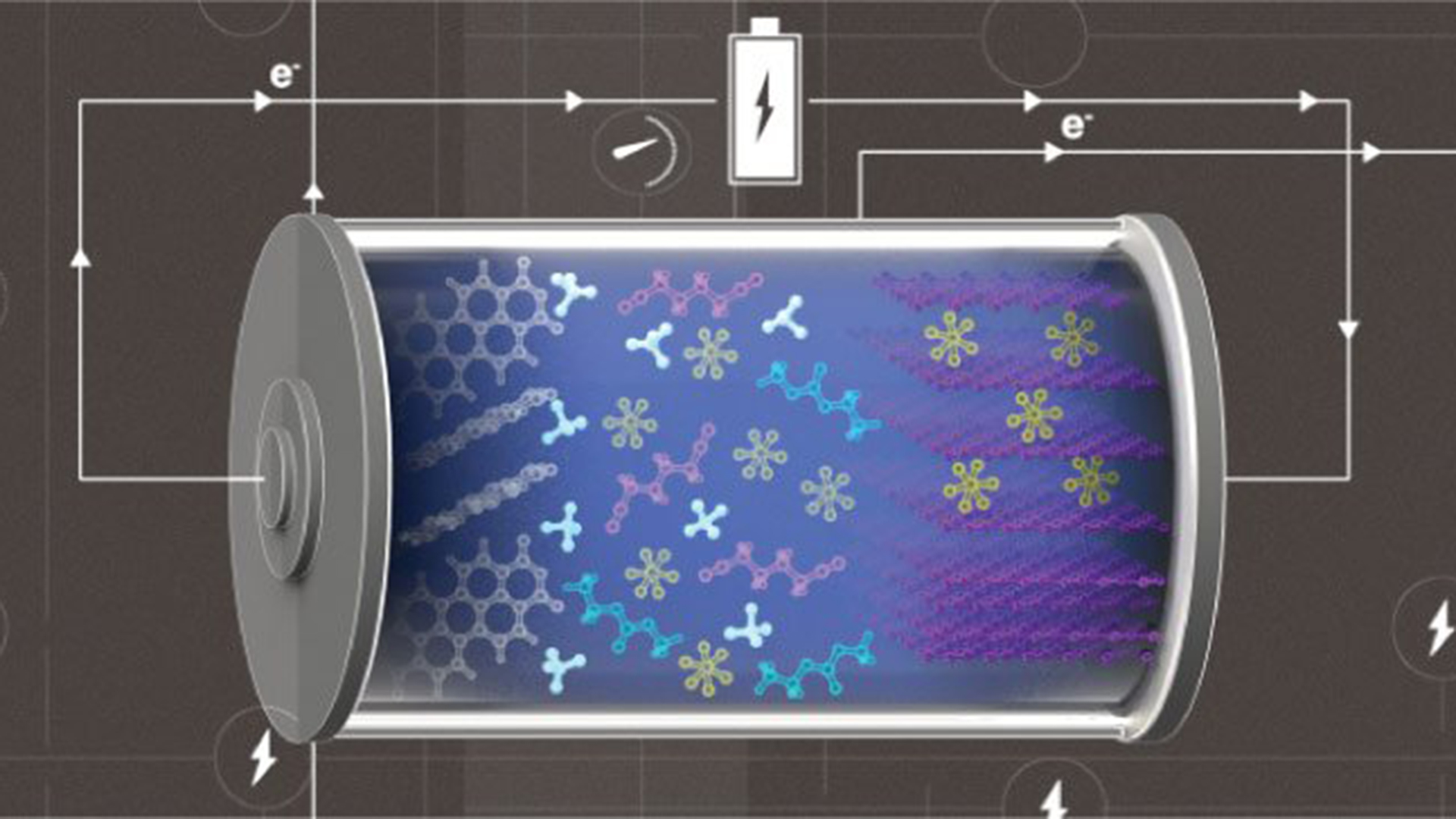
KAUST’s Zhiming Zhao commented: “We designed an electrolyte that is both antioxidative and antireductive by screening a series of solvents resistant to high voltage and also taking into account its reduction stability.
The antioxidative solvent predominantly solvated anions in the cathode reaction, whereas its antireductive counterpart created a solvation sphere around cations around cations in the anode reaction.
The novel battery significantly outperformed previous ammonium-ion-based analogues, recording an operation voltage of 2.75 volts.
Zhao explained: “It is now possible to develop high-energy nonmetallic ion batteries that can compete with metal-ion batteries. We are exploring anode materials with a higher capacity, which is crucial for improving the energy density.”
KAUST’s Husam Alshareef added: “To completely decarbonise the grid, the battery costs must significantly come down.”
The team’s groundbreaking ammonium-ion could be instrumental in lowering these costs.