Professor Edwin Bergin, of the Department of Astronomy at University of Michigan and Dariusz Lis at NASA JPL-Caltech, discuss the astrochemical origins of water on Earth and its evolutionary history
Water is the essential liquid of life, yet its origins are found in the pre-history of our planet – that is the birth of our Sun and its planetary system. The origins of water on Earth and its evolutionary history are at the beating heart of inter-disciplinary science from astrochemistry to geochemistry, to biochemistry.
In this article, we describe the origins of Earth’s water, beginning about ~1 million years prior to the birth of our Sun, and place its history within the interdisciplinary landscape that is wrapped around the mystery of the origin of life on our planet. Astronomical study is key facet of this landscape, pointing towards the need for new space-based observatories to search for water on other worlds and explore the supply of water to planets as they are assembled.
Icy Beginnings
The space between stars is not filled with water, or dihydrogen oxide (H2O), rather it is filled mostly by atomic hydrogen gas with 1 hydrogen atom per cubic centimetre. Other elements, in particular oxygen, are also found in atomic form with significantly lower abundance. Molecules, such as water, cannot form, as space is also filled with radiation from stars that readily dissociates water leaving elements as atoms. Also, a portion of elemental oxygen is locked up in silicate minerals in the form of particulate matter with a typical size of 0.1 microns (10-6 meters) that astronomer’s call stardust. This stardust forms during the late stages of stellar evolution, as stars return their envelopes, along with these tiny grains, to interstellar space. Stardust is important, as these grains are essentially the tiny seeds of Earth-like worlds floating in space.
Star birth begins when regions of interstellar space become over-dense by factors of a thousand or more, or in other words: gravity wins. When this occurs, two things happen nearly simultaneously. First, the effects of radiation become minimised as starlight is extinguished through direct absorption by stardust. This leads to the formation of molecules; stars are born in molecular clouds. Second, because of these molecules, the gas cools down from temperatures of ~1000 °K (623 °C) to only 10-20 °K (-263 to -253 °C). The first molecules to form in this very cold gas and dust are gaseous molecular hydrogen and carbon monoxide, and also solid-state water, or water ice. We know this is the case from astronomical study and from our own solar system.
On the astronomical side, we can observe molecules as ices and gas in regions of incipient star birth using telescopes on our planet. We most generally need to use observational platforms that are above our atmosphere, as water vapor in our atmosphere hinders astronomical observation of extra-terrestrial water. Examples are spacecraft such as the Submillimetre Wave Astronomy Satellite, Odin, the Spitzer Space Telescope, and the Herschel Space Observatory. These observatories, and subsequent theoretical analysis, have definitively demonstrated that hydrogen and oxygen react to form water ice via catalytic reactions on the surfaces of stardust prior to stellar birth [1] [2]. The reaction sequence to form water as gas and ice has also creatively been replicated in chemical laboratories on the Earth, confirming astronomical theories [3].
Within solar system bodies there is also a strong clue to the initial cold origins of water. All solar system water has an excess of deuterium relative to hydrogen. Deuterium is an isotope of hydrogen with a proton and a neutron (hydrogen only has a proton). Deuterium was created during the big bang and has a ratio to hydrogen of order 1 part in 105. This means for every 1 deuterium atom there are around 100,000 hydrogen atoms. Because Deuterium atoms are heavier, they form slightly stronger bonds than Hydrogen. Laboratory experiments and chemical theory have shown that this causes Deuterium atoms to be enriched within molecules with strong bond interactions, provided the temperature is below 30 °K [4]. All water in the solar system, in the Earth, meteorites, and comets bear this Deuterium enrichment, as shown in Fig. 1. This tells us that this water first formed at very cold temperatures that are found by astronomical observations to predominantly exist before our star is born [5].
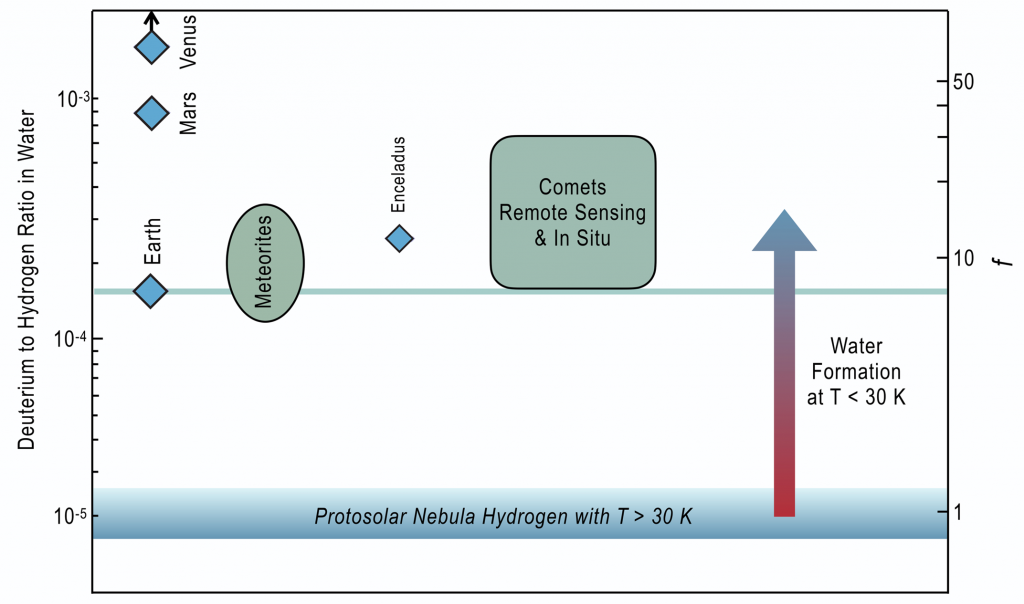
Deuterium to Hydrogen ratio in Water within various solar system bodies shown as the atomic ratio (left) and as an enhancement relative to nebular gas (right). Circle for meteorites illustrates the range of values seen in meteoritic material. The cometary box illustrates the same. The blue box at the bottom shows the expected ratio that was set by the deuterium abundance provided since the birth of the Universe. The arrow to the right represents the fact that the chemistry that produces these deuterium enrichments is activated at T < 30 K. Also shown are Venus and Mars (atmospheric average), which both have an elevated D/H ratio that bears additional information on the history of water in their atmosphere [6].
Delivery to a Baby Planet
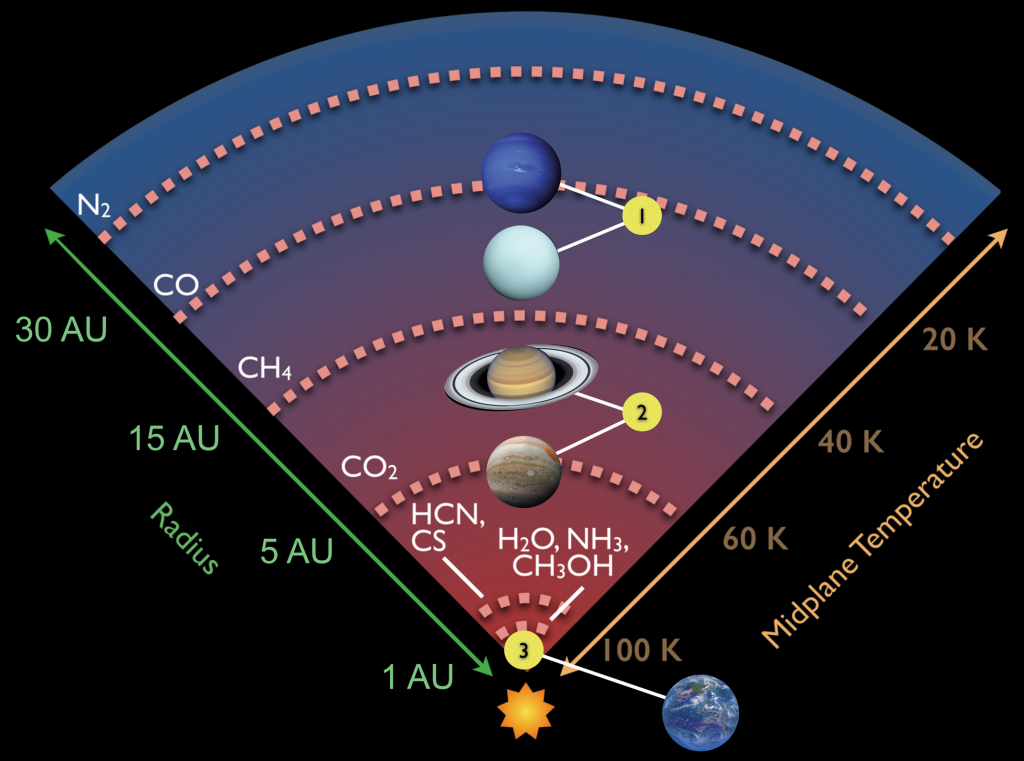
Water first forms as ice about 1 million years before the birth of the star. In our solar system, this was 4.6 Billion years ago. When gravity begins to win and the gas becomes denser and denser, the rotating cloud of gas begins to collapse. Due to the physics of gravitational collapse, this forms a star, our Sun, and a disk of gas and stardust. This disk is where planets are born and where water is incorporated as ice into the rocks as planets are assembled. A central aspect that governs the composition of forming bodies within the disk is its temperature. Simply put, it is hotter closer to the star, and colder further away. For water this means there is a phase transition at some distance from the star (called the iceline), where inside this point, water is found as vapour and beyond water exists as ice. This is illustrated in Fig. 2 where the (rough) gas to ice transitions for a variety of compounds are shown. The rocky cores of all gas giants (points 1 and 2) likely had substantial water content. In contrast, the Earth (point 3) formed inside the iceline where water is present as gas. Since the Earth formed from solids (both rocks and ices), it therefore received little water at birth.
Based on this, our current view is that the Earth initially formed dry. However, the young solar system is not a static place, as during its formation, the Earth was constantly bombarded by rocks that originated at a range of distances from the star. This includes bodies in the proto-asteroid belt, many of which likely contain water. For example, Ceres, the largest body in the asteroid belt, is thought to be of order 17% to 27% water by mass [7]. Alternately, ice-dominated bodies that formed near the gas-giants (i.e. comets) could have supplied the needed water. The D/H ratio of various bodies in the solar system provides a fingerprint that might be used to trace the supply of Earth’s water by using the Earth water D/H ratio as a metric. Until recently, meteorites, which are thought to originate in the asteroid belt, provided the closest match, with comets exhibiting higher D/H ratios, by a factor of two or even more. However, in 2010 the Herschel Space Observatory measured the D/H ratio in a Jupiter-family comet, finding a lower D/H ratio, comparable to that seen in Earth’s water [8]. It remains more likely that the Earth received most of its water from material that originated near the asteroid belt. This is because it is dynamically easier to deliver nearby material [9]; however, comets deliver more water by mass and a smaller number of impacts might therefore have greater effect. At present, our survey of cometary D/H ratio is constrained by measurements of only ten objects that sample only the tiniest fraction of the population, and thus substantially more work needs to be done to understand the full picture of the origins of Earth’s water supply and, by proxy, other habitable worlds.
Enabling Chemistry with Memory
The chemistry that leads to water formation in the depths of space is entirely abiotic in the sense that the conditions that exist in the medium favour water formation. This includes hyper-low pressures (less than a quadrillionth – 15 orders of magnitude of Earth’s surface air pressure) and temperature, with persistent sources of ionisation from cosmic rays that pervade galaxies. This leads to the ubiquitous presence of water ice in the solar system and, ultimately, the origins of water on Earth, and likely other terrestrial worlds in exo-planetary systems. Geophysical evidence suggests that water was present on the Earth’s surface as early as 4.3 Billion years ago [11]. When life, a chemistry with memory, subsequently originated is a matter of debate. Regardless, it is clear that the presence of liquid water as a solvent and facilitator is a critical molecule.
A Future of Discovery
Today, the astrophysical study of the origins of water on Earth lies at a crossroad. In the coming year, the James Webb Space Telescope (JWST) is scheduled to launch. JWST will observe water vapour emission in hundreds of planet-forming systems, thereby measuring their water content. However, because of the physics of the water molecule and the wavelengths JWST will observe (2–28 μm), this will only sample water close to the star, and will not trace the bulk water reservoir that is present a few Earth-Sun distances and beyond. To sample this water and reveal the full story, we must observe at longer wavelengths, towards what is called the far-infrared (30-600 μm). At these wavelengths, we access the lowest energy states of the water molecule that trace the bulk material of planet formation. Unfortunately, at present this avenue of exploration is now closed with the cancellation of two projects that had this ability. In 2020, an instrument in development for SOFIA (Stratospheric Observatory For Infrared Astronomy) called HIRMES was cancelled due to budgetary concerns. This was followed by the European Space Agency’s cancellation of SPICA, also for budgetary reasons. Without this information, our ability to understand the wealth of information expected about exoplanets, whether Jovian-size gas giants or Earth-analogues, will be hampered. As an important point, our own origin story – starting with the origins of water on Earth – is wrapped up in this.
Cutting edge science is expensive and NASA has a its flagship program with powerful transformational missions such as Hubble, Spitzer, or JWST. One potential future flagship is the Origins Space Telescope, which would have tremendous ability to observe much of the spectrum of water vapour and ice surveying the water content of over a 1000 still forming planetary systems. Origins would also be able to measure the D/H ratio of over 100 comets with high precision. If chosen, this would put us back on the trail of water and our origin.
However, Origins is one of four flagships that was presented to the US National Academy of Sciences Decadal Survey on Astronomy and Astrophysics. This decadal survey traditionally sets priorities for the coming decade. All four proposed flagships are equally worthy mission proposals and, at face value, probing diverse scientific frontiers would take more than 40 years. The next smaller category of completed astrophysics missions is a medium class explorer (MIDEX), with a budget cap of $290M. However, these new science frontiers require large telescopes and, in the case of the water trail, broad wavelength coverage and high spectral resolution. This is beyond the capabilities of an explorer. NASA’s planetary science division has Discovery and New Frontier class missions, with larger budget caps in the $0.5-1B range. However, this currently does not exist in astrophysics. A new Probe class of competed missions, as presented by NASA to the decadal survey, with a budget cap similar to Discovery or New Frontier, is the most promising way to answer the cutting-edge astrophysics science questions of today, such as those discussed here, in a timely and cost-effective manner.
The big questions of science, such as the mystery of life’s origins, enthral us all. As described above, astronomy has a role to play in this understanding. As scientists, we are grateful to the support from NASA, which has its mission to “reveal the unknown for the benefit of humankind”.
Acknowledgments:
Part of this research was carried out at the Jet Propulsion Laboratory, California Institute of Technology, under a contract with the National Aeronautics and Space Administration. EAB is also grateful to support from NSF AAG Grant #1907653 and NASA’s Exoplanetary Research (80NSSC20K0259) and Emerging Worlds (80NSSC20K0333) programs.
Authors
Professor Edwin (Ted) Bergin
Department of Astronomy
University of Michigan
+1 734 764 3441
ebergin@umich.edu
Dariusz C. (Darek) Lis
Jet Propulsion Laboratory
California Institute of Technology
+1 646 497 9374
dclis@jpl.caltech.edu
References
- E. A. Bergin and E. F. van Dishoeck, “Water in star- and planet-forming regions,” Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences, vol. 370, no. 1968, pp. 2778-2802, 2012.
- E. F. van Dishoeck, E. A. Bergin, D. C. Lis and J. I. Lunine, “Water: From Clouds to Planets,” in Protostars and Planets VI, Tucson, University of Arizona Press, pp. 835-858.
- E. F. van Dischoeck, E. Herbst and D. A. Neufeld, “Interstellar Water Chemistry: From Laboratory to Observations,” Chemical Reviews, vol. 113, pp. 9043-9085, 2013.
- T. J. Millar, A. Bennet and E. Herbst, “Deuterium Fractionation in Dense Interstellar Clouds,” Astrophyiscal Journal, vol. 340, p. 906, 1989.
- L. I. Cleeves, E. A. Bergin, C. O. ‘. Alexander, F. Du, D. Graninger, K. I. Oberg and T. J. Harries, “The ancient heritage of water ice in the solar system,” Science, vol. 345, no. 6204, pp. 1590-1593, 2014.
- T. Encrenaz, “Water in the Solar System,” Annual Review of Astronomy and Astrophysicsl, vol. 46, pp. 56-87, 2008.
- T. B. McCord and C. Sotin, “Ceres: Evolution and Current State,” Journal of Geophysical Research, vol. 110, no. E5, 2005.
- P. Hartogh et al., “Ocean-like water in the Jupiter-family comet 103P/Hartley 2,” Nature, vol. 478, no. 7368, pp. 218-200, 2011.
- A. Morbidelli, J. Chambers, J. I. Lunine, J. M. Petit, F. Robert, G. B. Valsecchi and K. E. Cyr, “Source regions and timescales for the delivery of water to Earth,” Meteoritics and Planetary Science, vol. 35, pp. 1309-1320, 2000.
- E. A. Bergin and L. I. Cleeves, “Chemsitry During the Gas-Rich Stage of Planet Formation,” in Handbook of Exoplanets, Springer, 2018.
- S. J. Mojzsis, T. M. Harrison and R. T. Pidgeon, “Oxygen-isotope evidence from ancient zircons for liquid water at the Earth’s surface 4,300Myr ago,” Nature, vol. 409, no. 6817, pp. 178-181, 2001.








