Dr Carlos Ziebert, head of IAM-AWP’s Calorimeter Center, KIT, explains how battery calorimeters and gas chromatography can be used for collecting and analysing venting gases of batteries.
There is an ongoing challenge between increasing the energy density of lithium-ion batteries (LIBs) and maintaining their safety level because increased energy density goes hand in hand with increased reactivity and violence of the energy release and thus a reduced cell safety. One reason is that the LIBs currently contain a liquid electrolyte that is flammable and can release toxic and flammable gases. Therefore, in addition to the achievable energy density that directly translates into the distance that an electric vehicle can drive and the cycling stability representing the battery’s lifetime, the safety of LIB is a decisive factor for the public acceptance and market penetration of this technology.
Recently, a new accelerating rate calorimeter (ARC), the EV+ from Thermal Hazard Technology (see Fig. 1), has been acquired and installed in the Battery Calorimeter Laboratory of the group Batteries – Calorimetry and Safety at the Institute of Applied Materials – Applied Materials Physics (IAM-AWP) of Karlsruhe Institute of Technology (KIT).
On the one hand, this new system provides an integrated infrared camera system that makes recording thermal images during abuse tests on LIBs possible. Thus it allows investigating the temperatures of the gases that evolve from the cells when they open up either at predefined pressures on their safety vents or randomly. On the other hand, the new system enables the detailed analysis of those venting gases in combination with a Clarus 690 Arnel 4019 gas chromatography (GC) system from Perkin-Elmer with two thermal conductivity detectors and a mass spectrometer (MS). This GC-MS system is jointly operated with two groups from two other IAM institutes, namely the Institute for Applied Materials – Materials Science and Engineering (IAM-WK) and the Institute for Applied Materials – Energy Storage Systems (IAM-ESS). The system was partly funded by the Strategy Funds of the Helmholtz Programme Storage and Cross-Linked Infrastructures.
Determination of critical safety parameters using an ARC
In the EV+ accelerating rate calorimeter, the cells’ self-heating, thermal stability, and the different processes leading to thermal runaway can be studied. During a mechanical, thermal or electrical abuse, the critical parameters and thresholds for the cells’ safe operation can be determined. The most influential parameters are the onset temperature, the internal pressure, the amount of heat released during thermal runaway and, last but not least, the volume of the released gases and their qualitative and quantitative composition. These gases are mainly produced by the electrolyte and its oxidation, reduction and decomposition reactions in interplay with the anode and cathode active and passive materials. The electrolyte in current LIBs is liquid and flammable. Due to the chemical content of the LIB, explosive or toxic gases can be released that represent a potential hazard due to their rapid and widespread distribution.

Gas sample collection using an ARC
On the top of their housing, cylindrical cells and prismatic hard case cells are usually equipped with so-called safety vents, which are predetermined breaking points that allow the overpressure release. Such overpressure is induced by the gas formed due to a mechanical, thermal or electrical abuse of the cell. Pouch cells, in contrast, do not provide a safety vent on the aluminium polymer compound foil that forms their housing. Thus they tend to open at any weakest point of their welding seeds. Typically this point is close to the current collectors, but it is not as well-defined as for the cell types with a metal housing. To allow the collection of the gases during the venting of the cell, one of the abuse tests mentioned above is performed in the ARC inside a pressure-tight canister.
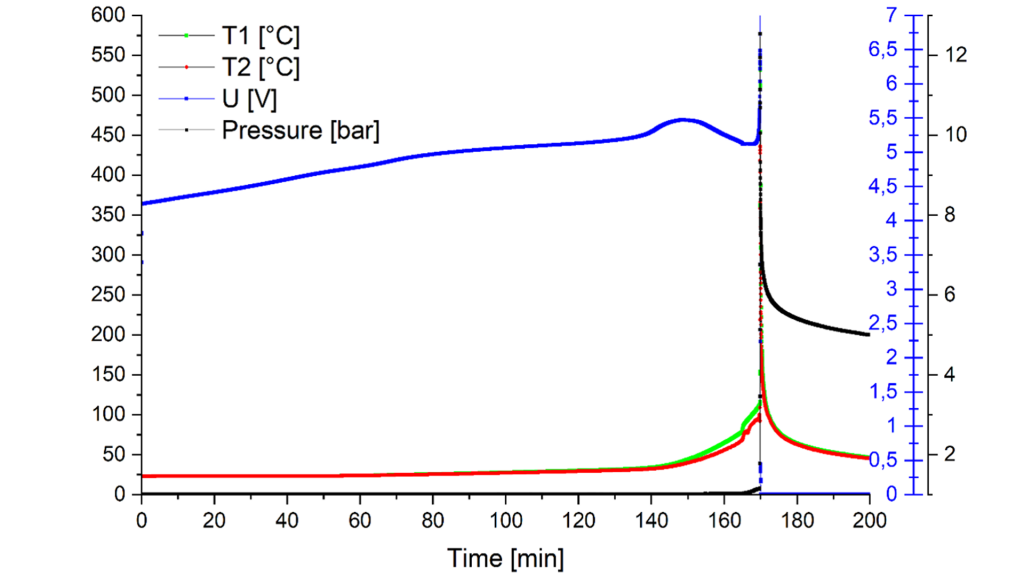
Fig. 2 shows as an example temperature, cell voltage and pressure curves during an overcharge test on a 14 Ah prismatic hard case cell. The curves in this example show that the cell venting occurs after 165 minutes. The cell goes into thermal runaway only five minutes later, which is indicated by the large temperature and pressure increase.
There are two approaches for gas sampling that have been elaborated in our lab: i) by a syringe and ii) by a gas mouse. The canister’s lid is equipped with a thin burst disc made from brass. After the abuse experiment, this burst disk can be pierced by the syringe’s needle, and gas can be drawn into the syringe, as shown in Fig. 3. For the second approach, the cylinder is connected via a small capillary to a Swagelok system with a gas mouse protected by a filter. This filter prevents particles from entering that could damage the delicate GC-MS system. When the cell vents during the abuse test, the gases are collected in the gas mouse.

Gas analysis by gas chromatography-mass spectroscopy
Directly after the test, the syringe or the gas mouse, respectively, are transported to the GC-MS system (see Fig. 4). Then the gas is released via a gas feeding system into the inlet of the GC and is analysed using the thermal conductivity detectors (TCD)). The columns in the GC part split up the different species and separate them by their different retention time. The retention time is dependent on the level of the interaction between the gas species and the stationary phase of the coating inside the GC columns. A stronger interaction leads to a longer retention time.
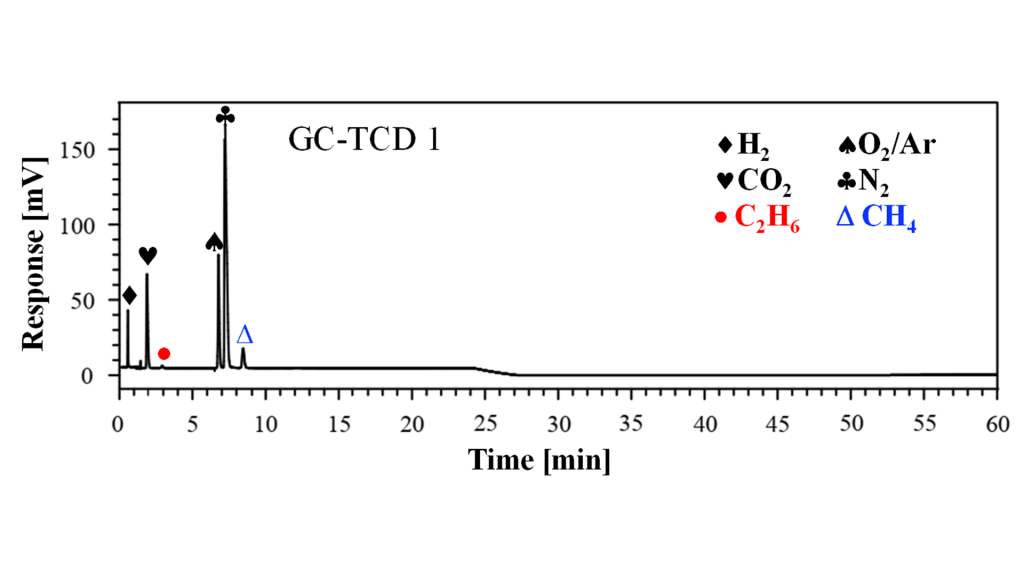
The interactions are governed by Intermolecular forces such as Van der Waals forces and dipole-dipole interactions. In Fig. 5, the thermal conductivity detector signal is depicted for the gas samples taken from the overcharge experiment shown in Fig. 3. It can be clearly seen that hydrogen, oxygen, carbon dioxide, nitrogen, methane and ethane have been found. If unknown components are detected, they can be further inspected using the mass spectrometer, which is also combined with the GC system. A calibration procedure will be elaborated in the following months to analyse the gas components qualitatively and quantitatively.
The AnaLiBa project
The new facility consisting of the EV+ ARC and the GC-MS system plays an important role in the project AnaLiBa (Analytics of Lithium-ion-Batteries). This project is funded by about €1.3m by the German Federal Ministry of Education and Research (BMBF) in the framework of the competence cluster Analytics/quality assurance (AQua). It began on January 1, 2021, runs for three years and is coordinated by the Fraunhofer Institute for Chemical Technology (ICT) in Pfinztal. The third partner is the Centre for Solar Energy and Hydrogen Research Baden-Württemberg (ZSW) in Ulm. The main objective in the AnaLiBa project is the joint method development of a standardised sample handling process for the gas analysis of small and large cells of different formats. This development first includes the described methods with the canister, secondly the introduction of a capillary into the cell, and thirdly a direct coupling of the ARC and an online mass spectrometer. The knowledge of the gas composition under the various cell abuse scenarios reveals the relevant decomposition paths and gives a hint which protective measures are necessary, e.g. to delay or prevent the cell fire caused by the ignition of the vent gases.
In the AnaLiBa project, different cell formats, different cell capacities and different application areas (high-energy/high-power cells) will be compared. The above-mentioned abuse tests will be performed on fresh cells. The changes in the gas composition, the cell behaviour, and the critical parameters will be studied after cyclic or calandaric ageing. Using the described analytical methods – which are to be optimised in the course of the project – cell safety mechanisms, their changes with ageing, and the influence of production faults can be investigated. All these studies serve both the mission of the AQua cluster and the innovation pipeline of the BMBF umbrella concept for the German battery research factory.
Please note, this article will also appear in the ninth edition of our quarterly publication.