A team of experts from Sensmet have developed µDOES® – a breakthrough technology helping to improve battery metal recycling and production processes.
New real-time analysis and process control technology in battery metal recycling and manufacturing will significantly improve the efficiency and effectiveness of hydrometallurgical processes. This increases productivity and reduces waste, whilst also helping to ensure end-product quality and purity.
A group of scientists and industry professionals at the company Sensmet have developed the µDOES® (Micro-Discharge Optical Emission Spectroscopy) on-line analyser, which offers real-time quantification of all battery and impurity metals. The recycling of used batteries is an extremely complex process where Sensmet’s solution provides a major step forward in black mass characterisation and process monitoring procedures that have traditionally relied heavily on time-consuming spot sampling and labour-intensive laboratory analysis. With a focus on robustness, real-time results, and ease-of-use, Sensmet’s µDOES stands out from analytical instruments such as ICP-OES – providing an ideal solution for industrial applications.
The level of automation and the swiftness of analytical methods are critical in enhancing production processes. Explaining the importance of this new technology for online monitoring, Sensmet CEO Dr Toni Laurila said: “The great strength of laboratory inductively coupled plasma-optical emission spectroscopy (ICP-OES) is its ability to accurately identify the atomic composition of complex samples. These instruments are extensively used for measuring metals in samples taken from metal manufacturing and battery metal recycling processes. But here’s the catch: even the most accurate ICP-OES lab analysis becomes irrelevant if it takes several hours to receive the results. By that point, the process has already progressed, rendering the lab results outdated.
“Our customers are consistently impressed with how effortless and convenient µDOES is to operate, and how accurate the results are. Also, the fact that the graphite and solid content in the leached black mass sample does not affect the measurement is highly valued. This is because the µDOES only requires sample dilution with demineralised water; it therefore eliminates the laborious sample preparation procedures that are required for ICP-OES analysis.”
How does it work?
The patented µDOES creates a micro-discharge (electric spark) in the diluted sample drawn from the process stream. This causes a microscopic volume of the fluid surrounding the spark to be flash-heated to 10,000°C so that molecular species in the spark are dissociated into atoms, which are excited to higher electronic states. When returning to their ground state, these atoms release excess energy by emitting light at their characteristic wavelengths. The µDOES measures this atomic emission spectrum to derive quantitative analysis of the metals in the sample.
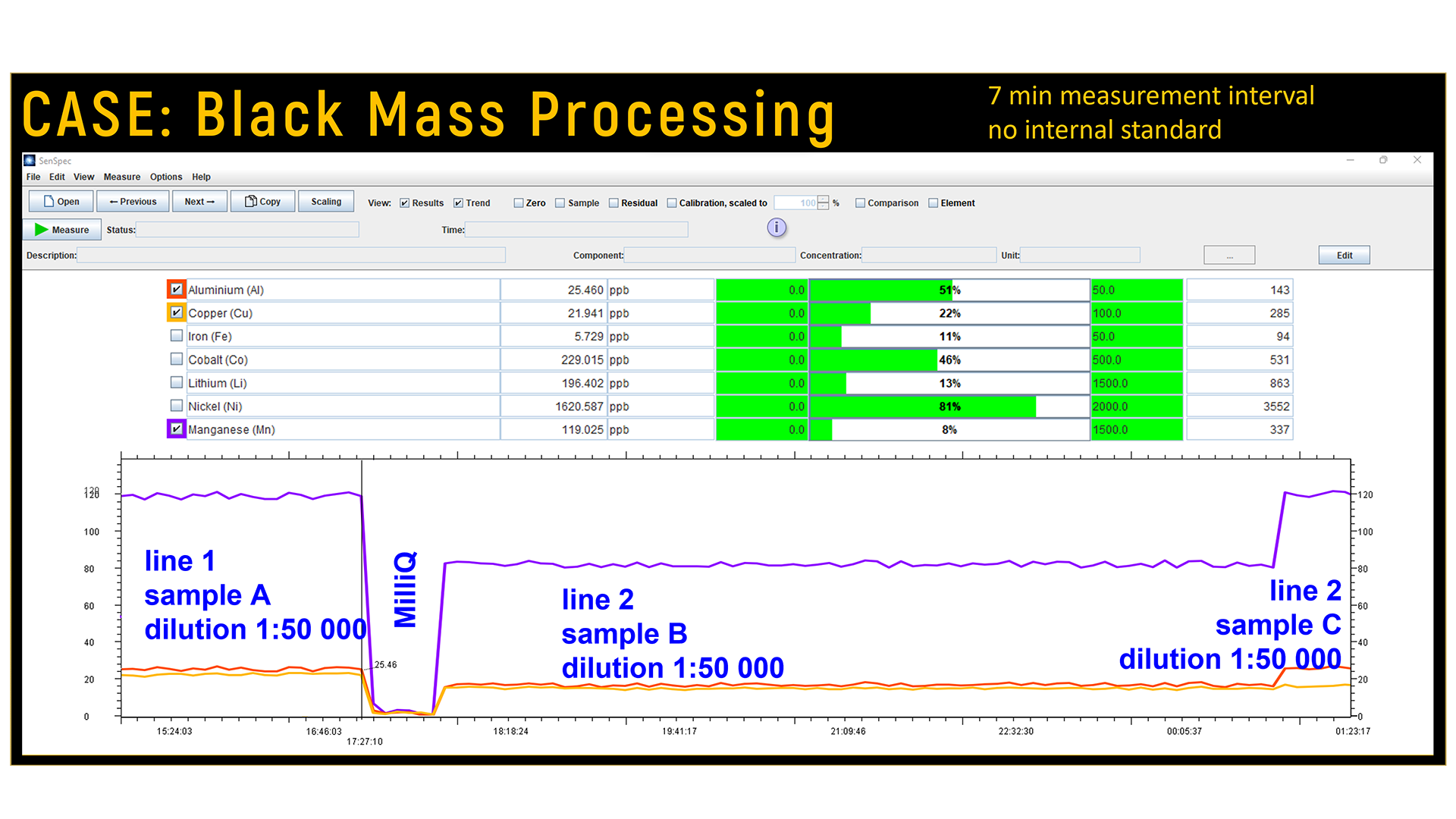
Data from the µDOES analyser are displayed instantaneously, showing the concentrations and trends for each metal. Results are also transferred digitally to users’ data management systems, which is crucially important because this enables real-time process control.
The µDOES instrument comes with user-friendly Senspec software, which not only manages data but also presents analysis results, time trends, and alarm levels for detected metals. Beyond simultaneous detection, identification, and quantification of metals, Senspec also adjusts for cross-interference effects to ensure accurate measurement results, even when analysing complex matrices with overlapping emission spectra.
Comparison with lab ICP-OES
In common with laboratory ICP-OES, µDOES is based on Optical Emission Spectroscopy (OES) with similar analytical performance. The key difference between ICP-OES and µDOES is their philosophy and design principles in plasma generation. Sensmet’s µDOES technology represents a rethinking of the principles behind ICP-OES, designed to meet the robustness requirements for continuous and fully automated industrial process measurement.
Aappo Roos, Chief Sales Officer at Sensmet, said: “Our customers have compared laboratory ICP-OES results with µDOES measurements, and have found that the difference is typically less than 10%. Interestingly, we have noticed that µDOES precision improves when the analyte concentration decreases.
“The µDOES has been designed to streamline the measurement process compared to traditional ICP-OES methods. It creates plasma directly in the diluted sample liquid, so no torch or costly carrier gas are needed. The robust design reduces maintenance requirements with the absence of components such as an RF coil, a spray chamber, and a nebuliser. Furthermore, unlike lab ICPs, the µDOES does not require internal standards to correct for matrix differences between the calibration standards and samples. If needed, customers can still run automated quality control samples at specified intervals to verify the accuracy of the system.”
Battery metal recycling
Global metal resources are finite and, with a surge in the deployment of electric vehicles (EVs), the volume of batteries reaching the end of their useful life will become enormous. Defunct batteries can be disassembled, so that the battery cells can be crushed and shredded to become ‘black mass’. Containing the remnants of the electrodes and electrolyte, the black mass is usually a mixture of carbon, metal oxides, and other residual materials that are difficult to separate and process.
The demand for battery metal recycling will inevitably escalate quickly. By closing the loop in battery metal lifecycles, the need for virgin materials is reduced. Thus, black mass – containing valuable metals such as cobalt, nickel, manganese, and lithium – represents a potentially valuable resource and is therefore the focus of recovery efforts in the recycling of used batteries.
Black mass is often leached to extract the valuable metals, however, the composition of the black mass can vary widely, depending on the specific battery design and manufacturing process, as well as the type of recycling previously applied. This makes the hydrometallurgical part of the battery recycling process most challenging. The resulting solutions must be constantly analysed to determine the composition and purity of the intermediate stages and recovered metals.
Real-time measurement has a crucial role in optimising the battery metal recycling process. By continuously measuring the concentrations of key metals like lithium (Li), manganese (Mn), cobalt (Co), nickel (Ni), copper (Cu), aluminium (Al), and iron (Fe), process managers are alerted to any anomalies early on, enabling them to improve both quality and efficiency.
Battery metal manufacture
With continuous monitoring, the efficiency of lithium manufacture can be greatly enhanced. Better dosing control and process management reduce waste and improve the final product quality, saving time and avoiding the costs of laboratory testing.
High metal purity is vital in battery production because impurities have negative impacts on battery performance. Impurities can result in reduced EV range, more frequent charging, decreased performance in cold temperatures, and increased risk of overheating. Thus, ensuring metal purity is critical for maintaining battery performance.
Other applications
Toni Laurila summarised the uniqueness of Sensmet’s µDOES technology: “Continuous on-site metals analysis provides benefits to many industries, but the battery industry will see the greatest gains. This technology allows for improved efficiency, lower costs, increased output, and better quality products. There is also potential for future use in pollution and environmental monitoring.”
Please note, this article will also appear in the thirteenth edition of our quarterly publication.







