Even if geological storage is the only option to tackle high levels of carbon emissions, CO2 electroreduction can be part of the solution to make the planet carbon neutral.
The global momentum to reduce CO2 emissions by replacing energy derived from fossil fuels with renewable electricity sources, such as photovoltaics or wind turbines, is steadily increasing. The associated challenges are twofold: identification of suitable technologies to reduce CO2 emissions; and determining appropriate chemical vectors to store the renewable energy (due to seasonal intermittency of renewables or for transport from off-grid regions with high renewable energy production).
Developing CO2 utilisation solutions such as CO2 electroreduction allows us to address both challenges simultaneously; because CO2 is used as a resource for the production of value added products using low carbon energy sources, towards a circular CO2 economy within a de-fossilised, sustainable industry.
In addition, this approach enables the storage of renewable electricity in form of easily manageable, transportable and storable high energy density hydrocarbons compatible with existing infrastructure. While CO2 utilisation can be used to achieve CO2 neutrality through significant reduction of atmospheric CO2 concentrations, CO2 geological storage is also needed according to the International Energy Agency.
Total’s ambition for CCUS
Total’s ambition is to have the globally recognised leadership in Carbon Capture Utilisation and Storage (CCUS) by 2035, with a two-pronged strategy to participate in climate change mitigation and prepare for new business opportunities. To achieve this goal, Total will position itself as an integrator of the full CCUS value chain, developing internal competencies, working in partnership and boosting innovative technologies to the market. In the context of CO2 utilisation, Total develops a portfolio of CO2-based products obtained via chemical and biochemical conversion with a low CO2 footprint, fitting into Total product portfolio and targeting mass markets that can have a significant impact on CO2 emissions in the long term.
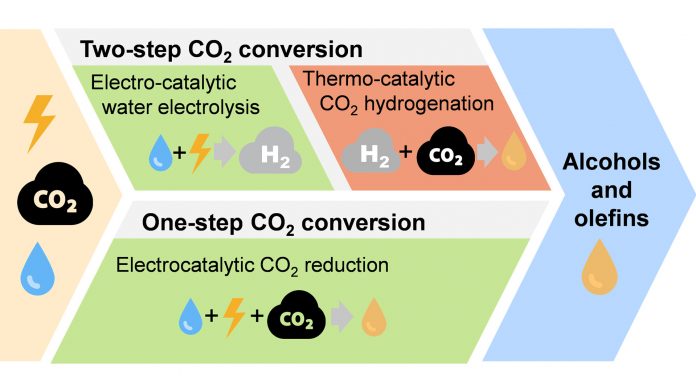
CO2 utilisation technologies are not yet fully developed or available at a scale that can sustain the global need for chemicals and fuels. Thus, from the perspective of the industrial development of CO2 utilisation, the most promising technologies must first be identified, assessed and compared in terms of their technical, economic and environmental feasibility. In this context, we identified two promising technologies for CO2 conversion: the one-step electroreduction of CO2 and the two-step conversion of CO2 with sustainable H2, either targeting olefins or alcohols as products.
While the two-step process requires H2 production via water-electrolysis followed by CO2 hydrogenation, the direct CO2 electroreduction can be done in one step, which is a conceptually advantageous in perspective of a potential industrial process. Both technologies can be readily compared, independently of uncertain electricity prices and potential incentives for CO2 emission reduction, as they start with the same resources (CO2, H2O, sustainable electricity) and allow to obtain similar products.
Today, the one-step CO2 electroreduction has a lower maturity (experimental proof-of-concept, technology readiness level (TRL) 2 to 3) compared to the two-step process (demonstrated in operational context, TRL 6), as direct CO2 electroreduction has attracted broader interest only recently. Hence, more fundamental understanding and development of CO2 electroreduction is needed to enable its evaluation, which is the basis for its comparison to the one-step and the two-step CO2 conversion technologies.
Using CO2 as a raw material
The CO2 electroreduction technology enables the user to directly transfer electrical energy to chemical energy in form of high energy organic compounds (such as olefins and alcohols) using CO2 as a raw material. Gaseous CO2 is introduced in the electrochemical reactor, where it reacts on the cathode catalyst with protons (H+) and high-energy electrons to form high energy products such as ethylene. The protons and electrons are formed at the anode catalyst. The catalysts are required to obtain reasonable production rates and product selectivity.
While the positively charged protons are transported from the anode to the cathode catalyst by the electrolyte and the membrane, the negatively charged electrons are transferred separately via an electrical circuit, which adds electrical energy to these electrons. This addition of electrical energy is required to be able to transform the low-energy-content CO2 to high-energy-content products like olefins and alcohols. As a result, for electrocatalytic processes, not only catalyst and reactor design as for thermocatalytic processes, but electrode, membrane and electrolyte design have to be understood, developed and associated into an integrated cell. Currently research and development on CO2 electroreduction focuses still on the cell and its constituting elements, while some upscaling efforts to stack several cells are attempted.
Together with its academic partners, Total pursues the fundamental understanding required for evaluation and at the same time develop application-oriented and next generation CO2 electroreduction catalysts. A record breaking catalyst for the electroreduction of CO2 to ethylene was recently developed in our collaboration at the University of Toronto and presented in the academic journal Science, featuring record activity (750 mA/cm2), high selectivity to ethylene (63% Faradaic efficiency) and high stability (> 150h). This catalyst will be the basis for University of Toronto’s competition entry, sponsored by Total, for the final round of the Carbon X-prize, a global competition to develop breakthrough technologies which convert CO2 emissions into valuable products.
CO2 electroreduction technology
The ambitious target is to scale the technology up to a unit capable of converting up to 100kg of CO2 per day within one year. This exercise will allow to swiftly identify critical steps in upscaling of the technology to a production unit level and thus facilitate evaluation and further development of the technology. In parallel, we focus on the deep understanding and the association of microkinetics (for catalyst at cathode and anode), mass- and charge transport (in electrodes, electrolyte, membrane and reactor) and heat transfer into an integrated multiscale model of the cell. This will enable to identify the performance limiting process elements and to optimise the cell geometry in perspective of potential scale-up.
Total works collaboratively on CO2 electroreduction with academic partners including College de France, University of Toronto, Stanford University, Lawrence Livermore National Laboratory, University of British Columbia and start-ups like OPUS12 and CO2 Solutions. The fast and significant development of the CO2 electroreduction technology towards industry relevant performances, as well as the leading position of Total and its collaborators in the field, is the basis for Total’s decision to further reinforce the efforts on leading the development of the CO2 electroreduction technology.
The joint understanding acquired via these partnerships will enable us to compare the one-step CO2 electroreduction and the two-step conversion of CO2 with sustainable hydrogen within the next few years. In case of continuing positive evaluation of the CO2 electroreduction technology, Total’s ambition is to develop this technology to the scale of a demonstration pilot (TRL 5) by 2035.
Encouraging a collaborative approach
Total is in contact with industrial equipment providers, experienced in electrochemical process design, to discuss the potential and challenges of scaling up the cell to an industrial production unit. Today the chemical industry is highly integrated in major complexes in part due to the need for separating complex feed streams. Converting pure or diluted CO2 streams selectively to a limited variety of products may allow for the creation of less complex plants; and thus potentially a more distributed production.
Due to the modularity of electrochemical processes, multiple units could either be connected in a cluster of centralised CO2 conversions; or units could be decentralised and situated close to similarly decentralised sustainable energy producers, including wind turbines and photovoltaic installations. This would enable a higher capacity utilisation of these installations; and therefore lower overall costs. However, the transport of CO2 and products to centralised industrial clusters will be easier compared to distribution to decentralised production sites, which brings further challenges to location and size of plants.
From a perspective of coupling CO2 conversion with decentralised renewable energy sources, technology must be developed to separate and convert CO2 from locally available CO2 streams, which are typically diluted. On the international scale, a perspective on system scale integration of production of chemicals via electrocatalytic processes – particularly with regard to CO2 electroreduction – needs to be developed (e.g. infrastructure for reactant transport (e.g. CO2) and energy management in a smart electricity grid), considering technical, economic and social boundary conditions. To tackle these challenges with a view to reducing CO2 emissions, Total encourages a collaborative approach and co-construction in CCUS research and development and CCUS full scale projects.
Total’s perspective on industrialisation of CO2 electroreduction
Historically, thermochemical processes have been economically favourable compared to their electrochemical counterparts for most products mainly due to the lower price of thermal compared to electric energy. Hence, electrochemical processes have only been developed to scale for products not easily obtained by thermochemical processes; for example in the chlor-alkali process for Cl2 production. Thus, electrochemical production processes for most products, including CO2-based products, are not yet developed and implemented at a scale that can sustain the global need for chemicals and fuels.
Nevertheless, today’s electrochemical commodity chemical production of chlorine and aluminium showcases the general feasibility of scaling up electrochemical production to a large scale. In perspective of the urgency to develop solutions to reduce CO2 emissions and assessing the use of renewable electricity for the production of CO2 based chemicals and fuels, the time horizon of conventional upscaling processes from the observation of basic principles (TRL 1) to extensive industrial implementation (TRL 9) in the chemical and fuel industry (typically 20+ years) has to be accelerated.
Currently existing plants and demonstrators would have to be scaled up into plants of several hundred MW (several hundred kT/y) for the production of CO2-based commodity chemicals; and to several GW plants (MT/y) for the production of CO2-based fuels and centralised integrated chemicals production clusters as we know them today. The development of CO2 electroreduction technologies is therefore a challenge spanning multiple scales. Starting from Total’s current focus on the constituting elements of the electrochemical cell (catalysts, electrodes, membrane, reactor cell), the next challenge of upscaling is to associate multiple cells into a cell stack, which will be the core of a production unit.
David Nevicato and Moritz Schreiber
CO2/CCUS Programme – Total SA
+33 (0)1 41 35 37 95