At the Research Priority Area Sustainable Chemistry of the University of Amsterdam, Dr Stefania Grecea is working to design molecular materials with combined physical and tunable chemical properties.
The ability to synthesise and tune the properties of molecular materials is a powerful driving force in the global scientific and technological community. Molecular materials allow the assembly of specifically designed molecules to obtain bulk structures with desired solid-state properties, enabling the development of materials with tunable chemical and physical properties.
Research Priority Area Sustainable Chemistry
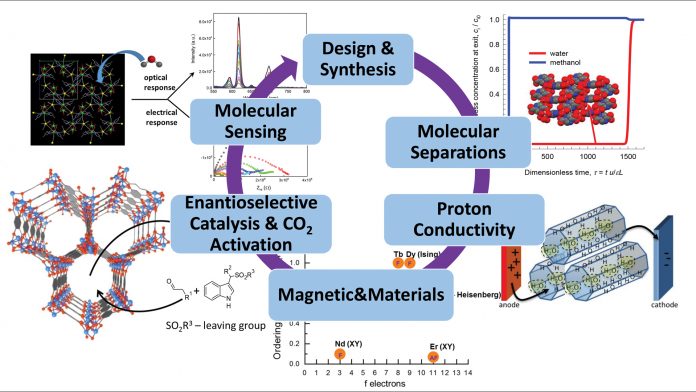
At the Research Priority Area Sustainable Chemistry of the University of Amsterdam, the Netherlands, Dr Stefania Grecea develops synthetic strategies to assemble well-designed molecular building blocks to combine in the same crystal lattice a set of properties that are difficult to achieve in a conventional inorganic solid. The methods developed target the designed synthesis of hybrid inorganic-organic materials and their composites which can be used in various applications, including molecular separations, molecular sensing, catalysis as well as proton-conductive materials for fuel cells.
My group designs novel materials which are assembled from metal ions or clusters of metal ions bridged across by organic molecules to form three-dimensional porous structures. These materials are also known as metal-organic frameworks (MOFs). A key property of these materials is their permanent porosity, which enables the reversible storage and release of guest molecules and also allows the differential recognition of molecules. Therefore, they can be used for molecular storage and separations. The spectroscopic properties of specific MOFs are sensitive to the entrance and/or release of the guest molecules to and from the pores and can therefore be used for chemical sensing.
A key property of the MOF materials for many practical applications is their long-term stability in humidity conditions. Therefore, one of the main goals in my research is to develop MOF materials which are highly stable in the presence of water. In doing so, my team employs synthetic methods which lead to flexible porous networks containing highly hydrophilic channels. Such materials are not only stable under humidity conditions, but in some cases they also bind water selectively, a key property for purification, humidity sensing, and proton conductive-related applications. Also, the presence of water molecules in the porous structure of MOF materials combined with their network flexibility enhances the binding and the activation of specific molecules, which is highly relevant for molecular storage and catalytic applications.
Molecular separations
In the chemical industry, the separation and purification processes are energy intensive and therefore highly costly. Such costs can be mitigated by developing materials that can enable efficient adsorptive separation. Separation technologies based on adsorption use porous materials which can separate various molecules using their physical properties such as kinetic diameter, polarisability, acid-base nature, co-ordinative properties, permanent dipole moment, and quadrupole moment. MOFs are ideal candidates as adsorbents for molecular separation because they offer the right balance between high adsorption capacity, operational life time, and energy requirement for regeneration. They are very interesting materials for molecular separations because their pore surfaces can be easily functionalised to tune the interaction with various molecules. They also have very high surface areas which enable optimised molecular separation as well as high selectivity and storage capacity.
For example, separating alcohol-water mixtures or removing traces of water from such mixtures remains one of the biggest challenges in several chemical industries, especially in biofuels purification. We have demonstrated that by combining lanthanide ions and specific organic molecules, we can obtain a stable microporous structure with hydrophilic one-dimensional channels which accommodates selectively water molecules. Using lanthanide ions, which have large co-ordination spheres and flexible co-ordination geometries, facilitates structural re-organisation of the porous structure without disrupting the overall framework. This makes the material highly robust, allowing multiple dehydration/hydration cycles. Unlike most MOFs, our material is also hydrothermally stable at high temperatures.
In another project, using a flexible organic linker in combination with alkaline metal ions, which are abundantly available, lightweight, and low-cost, we have shown that we can build highly flexible MOF structures which shrink upon the removal of guest molecules. For some MOFs, this specific behaviour results in large diffusional resistances towards N2 over CO2 molecules. Therefore, these materials have a very good CO2/N2 separation selectivity. Here, the specific design of the organic linker protects the metal ions from being attacked by water molecules and, therefore, our materials also have a very high humidity stability. This research is particularly relevant for CO2 mitigation, where CO2 can be removed and stored using adsorptive separation and storage methods. Using such methods, we physically adsorb CO2 using porous materials, thus providing an economically viable route for CO2 capturing. This method requires less energy for the regeneration of adsorbents in comparison to the currently used chemisorption methods.
Proton-conductive materials
MOF materials containing open channels which accommodate water molecules are preferable as proton-conducting materials. Such materials can be used to prepare proton exchange membranes that enable the migration of protons towards the electrodes in fuel cells. In MOF materials, the favourable path for proton transfer is the hydrogen-bonded network formed by lattice water molecules with each other and with the functional groups of the host framework. High water stability is paramount for such proton-conducting materials because they usually operate in a humid environment. The framework must retain numerous water molecules, and these must be mobile. This is because proton conductivity is predominantly determined by proton mobility and concentration.
To meet all these characteristics, we have developed a synthetic approach in which we use the self-assembly of molecular building blocks containing metal ions with flexible co-ordination geometries to form porous structures with open channels which accommodate water molecules selectively. The presence of multiple hydrogen-bonding interactions within the frameworks’ pores makes these materials one of the best proton-conducting MOFs.
Molecular sensing
MOF materials which bind water selectively are attractive candidates for humidity sensing. Their advantage is the large surface-to-volume ratios available for interaction with water molecules. This can increase the sensitivity and response speed of the sensors tremendously, as compared with conventional sensor devices. Moreover, we can tailor the size, composition, and shape of the materials and thus tune the sensitivity and the selectivity of the sensors. Importantly, these materials can be integrated into films and on specific surfaces, making them an ideal platform for designing new sensors.
In our research we develop lanthanide-based MOFs as optically responsive sensors because they have narrow emission and high colour purity resulting from the lanthanide ions. The key characteristic of these materials is the presence of the lanthanide ions in their structure. The water molecules or other guest molecules in the host MOF influence the optical properties of the lanthanide ions, including light absorption and emission profile. Therefore, we use these characteristics as sensing signals. The host-guest interactions within these MOFs allow the
pre-concentration of analyte molecules within the pores, which is responsible for highly sensitive detection. We can also achieve enhanced selective detection by inserting specific functional groups in the MOF structure to promote preferred analyte binding. Therefore, we can make various materials which afford the selective detection of various molecules.
A key advantage of our molecular building-block approach is that we can make materials which have combined physical properties, e.g. optical and electrical. This enables the combination within the same crystal lattice of porosity, optical, and electrical properties. Recently, we have demonstrated that our synthetic approach enables us to make highly reliable dual-mode humidity-sensing materials.
References
- http://hims.uva.nl/
- http://suschem.uva.nl/
- DOI: 10.1039/C7CC01122A
Dr Stefania Grecea
University of Amsterdam
Van’t Hoff Institute for Molecular Sciences
s.grecea@uva.nl