Thomas Heinbockel presents groundbreaking evidence that the endocannabinoid system plays a role in regulating neuronal activity in the main olfactory bulb.
The cannabis plant Cannabis sativa contains approximately 450 chemicals that are commonly referred to as cannabis.1 Cannabinoids are a group of substances found in the cannabis plant and include Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD), which are the main cannabinoids. Marijuana includes part of the cannabis plant that is mainly responsible for the effects on a person’s psyche. It turns out that our body produces the chemical substances endogenous cannabinoids (endocannabinoids), which act like THC in the brain and nervous system (the ‘brain’s own marijuana’).2 Our understanding of the endocannabinoid system is based on research advances in limbic system areas (e.g. the hippocampus and amygdala). The study of this signalling system in the olfactory pathway is still in its infancy.
Cannabinoid-based therapies in the treatment of various brain disorders and the role of endocannabinoids as neuroprotective agents are at the forefront of medical research. A better understanding of cannabinoid signalling has the potential to pave the way for developing cannabis-related substances as medications. My research team are investigating how the endocannabinoid system regulates our sense of smell and its relation to neural network activity in the olfactory pathway.
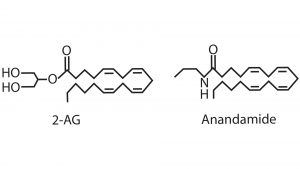
Endogenously produced cannabinoids are important neuromodulators
The human brain is equipped with a superb diversity of molecules that allow nerve cells to communicate with each other. The function of these neurotransmitter molecules have been demonstrated in many animal species, including humans. Prominent examples are the neurotransmitters ‘GABA’ and ‘glutamate’. Only fairly recently, another group of molecules has been demonstrated to be involved in brain signalling: the endocannabinoids. This is surprising, since the effects of exogenous cannabinoids as active ingredients in marijuana on human psyche and behaviour have been experienced by humans for centuries. The endocannabinoids are brain-produced modulators of neural signalling, which contrasts with their exogenous counterparts (THC, CBD) that are plant-derived and ingested into our body. The identification of endocannabinoids as signalling molecules and the functional mechanism of their action were only described approximately 20-30 years ago.3
The endocannabinoid system is comprised of its ligands, endocannabinoids and its receptors (CB1 and CB2). Endocannabinoids are fatty acid-derived signalling molecules that include N-arachidonoylethanolamide (anandamide, AEA) and 2-arachidonylglycerol (2-AG) (see Fig. 1). These two endocannabinoids bind to CB1 and have the same functional activity as marijuana. Based on the structural similarity between THC and endocannabinoids, THC is able to activate CB1 hijacks the brains communication system. The evolutionary origin of this communication system rests with endogenously produced cannabinoids that bind and activate CB1R. The endocannabinoid system was first discovered when activated by the plant-derived compound, THC.4 This is similar to the opioid system that can be activated either by endogenous ligands (such as endorphins), or by exogenously applied ligands (e.g. opioid drugs).
Cannabinoids in health and disease
Endocannabinoid receptors exist in all normal brains and have many essential brain functions when activated by their natural ligands, (such as motor behaviour, memory, and pain reception). Likewise, cannabinoids have therapeutic potential.5 Endocannabinoids show a neuroprotective role against acute excitotoxicity and facilitate functional recovery after brain injury. They regulate human airway function and provide a means to treat respiratory pathologies. The National Institutes of Health suggest that cannabinoids can be helpful in treating certain health conditions, such as treating certain rare forms of epilepsy, nausea and vomiting associated with cancer chemotherapy, and loss of appetite and weight loss associated with HIV/AIDS.1 Research studies indicate modest benefits of cannabis or cannabinoids for chronic pain and multiple sclerosis symptoms, whereas cannabis is not helpful for glaucoma.
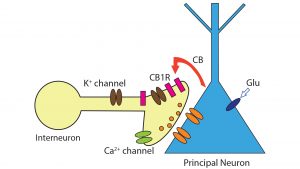
Retrograde signalling of cannabinoids
In the brain, endocannabinoids act primarily through the Gi/o-protein-coupled type 1 cannabinoid receptors (CB1 receptors, CB1Rs) and are synthesised from membrane lipids.6 Since endocannabinoids are lipid-based, they are able to diffuse through membranes. Anandamide and 2-AG were discovered in the early 1990s, while their functional role in neuronal communication remained obscure for years.2 Since their discovery, the role of endocannabinoids as retrograde messengers that suppress both excitatory and inhibitory transmission, has been well-established. Endocannabinoids mediate retrograde signals in the hippocampus, cerebellum, neocortex, amygdala, and main olfactory bulb.3 Termination of endocannabinoid signalling is accomplished by reuptake into both neurons and glia. Subsequently, anandamide and 2-AG are hydrolyzed intracellularly by fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL).7
CB1 is expressed in many different regions of the brain (such as the basal ganglia, and olfactory bulb). The receptors are mainly localised at presynaptic terminals where their activation modulates or decreases the release of presynaptic neurotransmitters (e.g. GABA and glutamate) via voltage-gated ion channels.8 Upon their release, endocannabinoids act at cannabinoid receptors on nearby presynaptic terminals to reduce the release of neurotransmitters, thus mediating a form of retrograde signalling (see Fig.2) . This is a distinctly different signalling mechanism to glutamate and GABA. These classic neurotransmitters are released by presynaptic neurons and activate receptors on postsynaptic nerve cells. The unconventional type of neuronal communication (mediated by endocannabinoids) is the Depolarisation-induced Suppression of Inhibition (DSI).2,8 In this communication system, a depolarised postsynaptic principal neuron releases endocannabinoids to regulate neurotransmitter release of presynaptic interneuron. Experimentally, a short rise in intracellular calcium concentration in a principal neuron (e.g., a tufted cell in the main olfactory bulb) results in a transient decline of incoming inhibitory signals in the form of GABA arriving from other neurons. During DSI, endocannabinoids travel from the postsynaptic neuron to the presynaptic GABA-releasing interneuron and turn off neurotransmitter release through activation of potassium channels and blockade of calcium channels.
Conventional chemical synaptic signalling between two neurons involves activation of a presynaptic neuron resulting in transmitter release and subsequent activation of the postsynaptic neuron. When the interneuron is activated, it releases the inhibitory neurotransmitter GABA and inhibits the pyramidal cell. During DSI, the inhibitory input onto an activated pyramidal cell is reduced. It is the endocannabinoids that were found to act as retrograde signalling molecules that mediate communication between postsynaptic pyramidal cells and presynaptic inhibitory interneurons and evoke the reduction in GABA release. Since endocannabinoids are lipids, they do not diffuse over great distances in the watery extracellular environment of the brain. Rather, DSI acts as a short-lived local effect that enables individual neurons to disconnect briefly from their neighbours and encode information.8,9 DSI was mimicked by activating cannabinoid receptors whereas blockade of cannabinoid receptors prevented DSI.10 A corresponding phenomenon, DSE, Depolarisation-induced Supression of Excitation, mediated by retrograde action of endocannabinoids, was identified at cerebellar excitatory synapses.11 DSI is considered a mechanism that allows individual neurons to disengage from other cells in their network so they can encode information.9
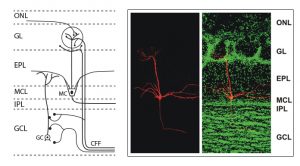
The olfactory system
For several years, we have worked on determining the role of endocannabinoids in the olfactory pathway, specifically in the main olfactory bulb, the first central relay station for processing of olfactory information that comes from the nose.12 The main olfactory bulb offers an ideal platform for investigating how the endocannabinoid system modulates a functional neural network to achieve an integrated outcome. The main olfactory bulb directly receives sensory input from the nasal epithelium. As we breathe in air, odour molecules activate olfactory receptor cells in the nose which send electrical signals (nerve impulses) to the main olfactory bulb. The main olfactory bulb receives strong centrifugal cortical input from higher order brain structures. This structural organisation makes the main olfactory bulb experimental preparation significantly different from hippocampus, amygdala, neocortex, and cerebellum to address functional questions of cannabinoid receptor-mediated modulation in the brain.
Our studies have a high potential for demonstrating novel and specific actions of cannabinoid receptors in the olfactory pathway. One possible result of cannabinoid receptor activation in the main olfactory bulb might be an increased response of its output neurons, the mitral cells, to weak stimulus input at the expense of loss of contrast. Contrary to several other studies that describe mechanisms of increasing contrast enhancement in the main olfactory bulb to improve odour detection, our data suggest that the endocannabinoid system might have the opposite effect.13-15 This is a novel idea for olfactory processing, and it is specific and relevant for olfaction. However, it is widely believed that contrast enhancement through lateral inhibition facilitates odour discrimination. The endocannabinoid system could help odour processing because it might be of behavioural/adaptive advantage to increase an animal/ human’s ability to perceive smells without detecting every specific odourant. An analogy might be the processing of fear-inducing stimuli in the amygdala. The amygdala receives sensory information through a fast, direct subcortical as well as a slower, indirect cortical pathway. The fast subcortical pathway helps us to respond quickly to dangerous stimuli at the expense of receiving impoverished stimulus information, while the slower cortical pathway resolves fine stimulus details.
Alternatively, it might be related to the dangerous effects of drug abuse. Marijuana is considered a psychoactive drug. Drugs such as marijuana generally increase or alter sensory perception (euphoric high) and strengthen the emotional relevance of environmental signals. The known marijuana effects include an altered state of consciousness, greater enjoyment of food taste and aroma, and increased sensuality. These subjective effects might come at the expense of clearly distinguishing the detailed nature of the stimulus, e.g., drug-induced distortions exist in the perception of time and space. At higher doses, marijuana may even lead to altered auditory/visual illusions. Our studies illustrates for the olfactory system, at the cellular level, the known effects of marijuana and possibly other drugs on behaviour and psyche.
The major rationale for our studies is that detailed knowledge of basic cannabinoid signalling mechanisms in the brain is needed at the cellular level to understand the properties of this new modulator system. Our studies provide answers to a number of those fundamental questions regarding the role of endocannabinoids at the cellular level and their relevance for synaptic processing in a sensory system that offers unique and distinct advantages for the study of the endocannabinoid system. Our new findings might explain the properties of marijuana at the cellular level through cannabinoid-mediated synaptic processing in the main olfactory bulb. We gain insight into the neuronal processes that take place after exposure to cannabinoids and how, even temporarily, neural circuits are driven into an altered state and changes manifest themselves as a result of the action of cannabinoids. These insights might eventually lead to a better understanding of drug addiction and could pave the way for new treatment strategies to prevent the use of drugs.
Endocannabinoids in olfactory signalling
Despite the relevance of the endocannabinoid system for other brain structures as well as human behaviour, the role of this transmitter system for odour processing largely awaits investigation. One of the emerging principles of synaptic processing in the main olfactory bulb, is the dominance of modulatory input: the relay from the nose to key output neurons, mitral cells and tufted cells, and from these cells to higher order olfactory centres is strongly regulated by local neural circuitry in the main olfactory bulb, including inhibitory GABAergic interneurons and by centrifugal inputs to the main olfactory bulb from other brain areas. Therefore, a pressing issue in the organisation and operation of the olfactory system is the functional significance of modulatory input (i.e. the endocannabinoid system). Our studies address the influence of the endocannabinoid system on synaptic processing in the main olfactory bulb. Not only will these studies have a significant impact on our understanding of the neurophysiology of the olfactory system but they will also allow us to design pharmacotherapeutic approaches to address substance use disorders and advance our ideas about cannabinoid-based therapies in the treatment of various neurological and neuropsychiatric disorders.
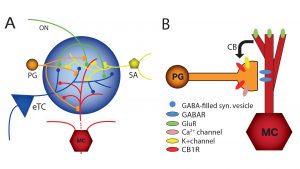
Although DSI was originally observed in the hippocampus, we found that it also occurs in the main olfactory bulb.14,16 In the main olfactory bulb, GABAergic interneurons in the input layer, the periglomerular cells, express cannabinoid receptors, CB1. The activation of CB1 in these interneurons directly and potently inhibits them and reduces their output of inhibitory GABA neurotransmitter. These interneurons form inhibitory GABAergic dendrodendritic (reciprocal) synapses with output neurons, tufted cells and mitral cells (see Fig.3). Mitral and tufted cells in turn form excitatory (glutamatergic) synapses on these interneurons. Our data also show that endocannabinoids mediate strong effects in mitral and tufted cells, and suggests that endocannabinoids are modulators of feedforward inhibition of output neurons. The interneurons show an inverse response pattern to cannabinoid receptor activation compared to mitral and tufted cells, indicating that cannabinoid receptors indirectly regulate mitral and tufted cell activity as a result of interneuron activation. We propose that CB1 directly regulates interneuron activity to control GABA release, which, in turn, regulates mitral and tufted cell activity (see Fig.4). This disinhibition of mitral and tufted cells may serve to make these neurons more responsive to sensory inputs and can be an important neuromodulatory mechanism in olfactory processing.3,14
Our results presented the first evidence that the endocannabinoid system plays a role in regulating neuronal activity in the main olfactory bulb. This regulation manifests itself in different neuronal cell types and through direct as well as indirect mechanisms. Our data indicates that sensory input to the brain, is subject to modulation as a result of cannabinoid receptor activation. Activation of cannabinoid receptors in the main olfactory bulb functions to increase sensitivity of output neurons, the mitral and tufted cells, through suppression of inhibition by interneurons. As a result, mitral and tufted cells become more responsive to synaptic input and odour stimulation. Functionally, the effect of cannabinoids in the main olfactory bulb is a transient regulation of sensory input from the nose to the main olfactory bulb and output to higher order olfactory centres in the brain. CB1 dynamically modulates olfactory processing in main olfactory bulb and shape synaptic output to higher olfactory centres. Cannabinoid receptors are known to be involved in brain function and dysfunction.
Smell, hunger and olfactory threshold
Intriguingly, research groups in both France and America have shown that cannabinoid signalling in the main olfactory bulb is implicated in regulating appetite and olfactory threshold through centrifugal fibre input to another type of inhibitory interneuron (granule cells), as a means of cortical feedback to the main olfactory bulb.17,18 Feeding behaviour is a physiologic process that can be regulated by the neuromodulatory effects of endocannabinoids in the olfactory system. In this regard, CB1 can cause fasted mice to increase their feeding behaviour (hyperphagia) through an increased detection of food through olfactory mechanisms.17 The activation of CB1 in the main olfactory bulb was the reason of increased feeding to occur after fasting. They further showed that the CB1 control over feeding behaviour was of the olfactory corticofugal circuits. These important findings link hunger, olfaction and feeding behaviour by a mechanism involving endocannabinoid-mediated neuromodulation of synaptic transmission in the main olfactory bulb.
Several other recent studies have determined how processing of sensory information in the main olfactory bulb is shaped by massive centrifugal, or feedback, projections from higher cortical areas.19,20 For instances, researchers examined the role of the endocannabinoid system in regulating centrifugal input to the main olfactory bulb.18 The authors observed that CB1 mediates widespread suppressive effects on synaptic transmission at centrifugal fibre synapses onto interneurons in the main olfactory bulb. Centrifugal fibres can be either inhibitory or disinhibitory of mitral cells depending on circuit activation. Cannabinoid receptors can bidirectionally change the ratio of inhibition and disinhibition of mitral cells through their effects on centrifugal fibres.
The sense of smell relates not only to the identification of odours as being pleasant or unpleasant, but also impacts a wide array of life functions.21 Recent advances in neurobiology demonstrate that despite its relatively simple organisation, the processing within the olfactory system is deceptively complex with its many sources of intrinsic and extrinsic neuromodulation. The dominance of modulatory input is an underlying principle of synaptic processing in the main olfactory bulb, particularly neuromodulatory inhibition as a prominent regulator of neural activity. The endocannabinoid system exemplifies this, in the form of lateral inhibition and recurrent inhibition through retrograde signalling. The widespread expression of endocannabinoids and their receptors throughout the central nervous system and their distinct effects on neuronal signalling is indicative of an underlying important role for endocannabinoids also in these systems. Therefore, it is very likely that, in the future, the endocannabinoid system will be used even more heavily as a target for medical interventions.
References
- https://nccih.nih.gov/health/marijuana-cannabinoids
- Nicoll, R., and Alger, B.E. 2004. The brain’s own marijuana. Sci Amer 2004; 291:68-75
- , T, and Wang, Z. 2016. Cellular Mechanisms of Action of Drug Abuse on Olfactory Neurons. Int. J. Environ. Res. Public Health 2016, 13, 0005
- Ameri, A. 1999. The effects of cannabinoids on the brain. Prog Neurobiol 1999; 58:315-348
- Heinbockel, T. 2014. Neurochemical communication: The case of endocannabinoids. In: Neurochemistry. Thomas Heinbockel (ed), ISBN 978-953-51-1237-2, Rijeka, Croatia, InTech Open Access Publisher, ch. 6, p 179-198
- Di Marzo, V., Melck. D, and Bisogno, T. et al. 1998. it Endocannabinoids: endogenous cannabinoid receptor ligands with neuromodulatory action. Trends Neurosci. 1998 Dec;21(12):521-8. PMID: 9881850, DOI: 10.1016/s0166-2236(98)01283-1
- Muccioli, G.G. 2010. Endocannabinoid biosynthesis and inactivation, from simple to complex. Drug Discov Today 2010; 15:474-483
- Alger, B.E. 2002. Retrograde signalling in the regulation of synaptic transmission: focus on endocannabinoids. Prog Neurobiol 2002; 68:247-286
- Alger, B.E. 2012. Endocannabinoids at the synapse a decade after the dies mirabilis (29 March 2001): what we still do not know. J Physiol 2012; 590.10:2203–2212
- Ohno-Shosaku, T., Maejima, T., and Kano, M. 2001. Endogenous cannabinoids mediate retrograde signals from depolarized postsynaptic neurons to presynaptic terminals. Neuron 2001; 29:729-738
- Kreitzer, A.C, and Regehr, W.G. 2001. Cerebellar depolarisation-induced suppression of inhibition is mediated by endogenous cannabinoids. J Neurosci 2001; 21:RC174
- Heinbockel, T. 2019. Understanding the olfactory system. Research Outreach 109: 18-21. doi: 10.32907/RO-109-1821
- Petzold, G.C., Hagiwara, A., and Murthy, V.N. 2009. Serotonergic modulation of odour input to the mammalian olfactory bulb. Nat Neurosci. 2009 Jun;12(6):784-91. doi: 10.1038/nn.2335. Epub 2009 May 10
- Wang, Z.J., Sun, L., and Heinbockel, T. 2012. Cannabinoid receptor-mediated regulation of neuronal activity and signalling in glomeruli of the main olfactory bulb. J Neurosci., 32(25):8475-9. doi: 10.1523/JNEUROSCI.5333-11.2012
- Wang, Z.J., Hu, S.S., and Bradshaw, H.B. et al. 2019. Cannabinoid receptor-mediated modulation of inhibitory inputs to mitral cells in the main olfactory bulb. J Neurophysiol 122:749-759, doi: 10.1152/jn.00100.2018
- Wilson, R.I., and Nicoll, R.A. 2001. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature 2001; 410:588-592
- Soria-Gómez, E., Bellocchio, L., and Reguero, L. et al. 2014. The endocannabinoid system controls food intake via olfactory processes. Nat Neurosci. 2014 Mar; 17(3):407-415. doi: 10.1038/nn.3647
- Pouille, F., and Schoppa, N.E. 2018. Cannabinoid Receptors Modulate Excitation of an Olfactory Bulb Local Circuit by Cortical Feedback. Front Cell Neurosci. 2018 Mar 2;12:47. doi: 10.3389/fncel.2018.00047. eCollection 2018
- Mazo, C., Lepousez, G., and Nissant, A. 2016. GABAB Receptors Tune Cortical Feedback to the Olfactory Bulb. J Neurosci. 2016 Aug 10;36(32):8289-304. doi: 10.1523/JNEUROSCI.3823-15.2016
- Rothermel, M., Carey, R.M., and Puche, A. et al. 2014. Cholinergic inputs from basal forebrain add an excitatory bias to odour coding in the olfactory bulb. J Neurosci. 2014 Mar 26;34(13):4654-64. doi: 10.1523/JNEUROSCI.5026-13.2014
- Harvey, J.D, and Heinbockel, T. 2018. Neuromodulation of Synaptic Transmission in the Main Olfactory Bulb. Int J Environ Res Public Health. 8;15(10). doi: 10.3390/ijerph15102194
Thomas Heinbockel, Ph.D.
Professor
Department of Anatomy, Howard University College of Medicine
+1 202 806 0058
theinbockel@howard.edu
https://medicine.howard.edu/graduate-programs/anatomy