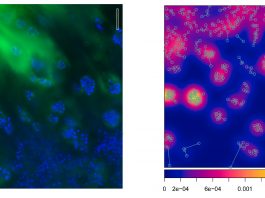
Dr Karine Anselme and Professor Maxence Bigerelle’s groups are continuing to collaborate with clinicians and companies in the development of more biocompatible and safe breast implants in the future.
For more than 50 years, surgeons and companies have been developing breast implants for aesthetic and reconstructive purposes. Already five different generations of implants have been developed in an empirical way in order to achieve either round or anatomically shaped implants. It can be noted that the chemical composition of breast implants does not vary much between the different models of implants currently on the market. Most of them are composed of an outer silicone envelope, often textured and filled with a gel, also made of silicone. The greatest difference between these implants is in accordance to their surface texture which can range from a completely smooth surface to a highly textured surface (see Fig. 1). This texture is necessary for adhesion to the breast tissue, particularly for anatomically shaped implants, and it also limits the contraction of the fibrous capsules that naturally form around the implants. This texture is obtained by different processes and results in textures of varying depth and morphology.
During the past 50 years, some scandals have revealed a lack of control surrounding the processes used for manufacturing and texturing breast implants. More importantly, there has been a lack of knowledge by the manufacturers and the clinicians about the influence of surface texture on biological responses. Very recently, a new scandal has emerged related to macro-textured implants that have shown a significant risk of developing an immune cell cancer, the breast implant associated anaplastic large cell lymphoma (BIA-ALCL). In 2019, this risk justified the withdrawal of the CE marking and the authorisation to sell on the European market the best-selling brand of macro-textured implant (BIOCELLTM, Allergan). Moreover, in France, all macro-textured implants present on the market have even been recalled regardless of their manufacturer. This recall was carried out following the joint results of the teams of Dr Karine Anselme and Prof Maxence Bigerelle, authors of this article.
Indeed, in 2015, following the increase in BIA-ALCL cases and the over-representation of macro-textured implants in the statistics, the French company SEBBIN, which sell among other textures the same type of implants, approached the authors of this article to begin a research project with them. The objective of this project was to characterise in depth the texture of implants currently on the market, to propose a new classification of implants based on their texture parameters, to qualify the biological response to these macro-textured implants, and finally, to determine the risks linked to the wear of these macro-textured implants in the tissues.
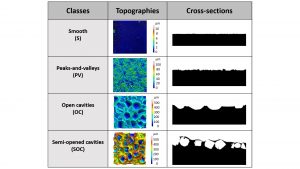
A multi-scale analysis of the surface texture of implants
Depending on the manufacturer, the descriptions of the implant surfaces and the name given to them vary greatly, even if their texture looks identical and is obtained by a similar process. Moreover, the international standard (ISO 14607:2018) for measuring these textures is incomplete and proposes to use unsuitable measuring instruments for certain textures, in particular for the previously cited problematic macrotexture’s morphology. In the course of this work, Bigerelle’s group, internationally recognised in topography analysis, carried out a multi-instrument analysis of the entire range of breast implant textures present on the European market.1 It showed that in order to characterise all of these implants correctly, it was essential to use two tools and not just one as proposed in the standard. By using the right tools, it is then possible to carry out an analysis at all scales of the implant surfaces. This information is essential for the understanding of their interaction with biological objects of different sizes (such as biomolecules, cells or breast tissue).
Proposal for a new classification of breast implants based on their texture
A robust statistical analysis of the topographic measurements obtained with these tools at different scales and on all the representative textures of the implants currently on the EU market was performed. As a result, it was possible to group the different textures into four categories and to name them according to the macroscopic aspect of their surface seen on a cross section (smooth, peak and valley, open cavities, semi-opened cavities) (see Fig. 2). It should be noted that the implants named so far macro-textured all belong to the semi-opened cavities category. For the first time, we have been able to propose a classification of breast implants statistically demonstrated on the basis of two different parameters, in contrast to the arbitrary classifications proposed in the literature and based on a single parameter. It would be desirable that this classification is now considered for the international standard.
The biological behaviour of tissue around breast implants
In order to validate this classification, Anselme’s group have developed a molecular approach to analyse the genes expressed by the cells present in the fibrous capsules around implants of different textures. They focused on healthy, uncontracted capsules and were able to show that even in patients with no clinical signs, the capsule cells around macro-textured implants (the category of implants with semi-opened cavities) showed overexpression of genes related to the organisation of the extracellular matrix and the inflammatory reaction compared to other types of implants, even more than 10 years after implantation. This work, published at the end of 2019 in the best clinical journal in the field (Plastic Reconstructive Surgery), suggests that the texture of the implants implicated in the recent scandal may indeed be the cause of cancers due to its ability to maintain long-term inflammation.2 It must be understood how these semi-opened cavity textures can induce such prolonged inflammation – the mechanical wear of the particular texture of these implants and the release of silicone debris into the surrounding tissues could be questioned.
The wear of macro-textured implants after implantation
While it is impossible to mimic in vitro the mechanical wear that implants undergo inside the breast, Bigerelle’s team preferred to compare the texture of different implants of the EU market before and after implantation. The results showed a significant wear of all textures but with a more important formation of debris for the semi-opened cavity textures due to the degradation of the silicone “caps” over the cavities. This release of debris around the implants involved in this latest scandal could explain the maintenance of peri-implant inflammation over several years and could potentially be the cause of lymphomas.
Innovation: the future of breast implants
These latest results demonstrate that it is essential that all players in the breast implant industry, including manufacturers, clinicians and notified bodies, understand that implant texturing is a critical point in the manufacture of breast implants. Silicone, even though it has been considered bio-compatible for many years, can become extremely dangerous biologically if it is shaped in such a way that it produces a fragile texture that may release debris after implantation. It is therefore obvious that in order to meet surgeons’ requirements for immediate post-implant attachment of implants, it will be essential to design safer textures in collaboration with specialists in the biological effects of texture such as the groups of Bigerelle and Anselme.
Finally, now that the methods for characterising textures and classifying implants have been defined, it appears essential to understand more precisely the biological mechanisms involved in the tissue integration of breast implants in order to develop the breast implants of the future, whether they are stable or degradable over time. Anselme and Bigerelle’ groups are ready to continue to contribute in collaboration with clinicians and companies in the field of developing more biocompatible and safe breast implants in the future.
References
1 Garabedian, C. et al. 2017. A multi-topographical-instrument analysis: the breast implant texture measurement, Surface Topography: Metrology and Properties. 5(2) 025004
2 Brigaud, I. et al. 2019. Surface texturation of breast implant impacts extracellular matrix and inflammatory gene expression in asymptomatic capsule, Plastic Reconstructive Surgery. Dec 23, doi: 10.1097/PRS.0000000000006606
Dr Karine Anselme
IS2M UMR UHA/CNRS 7361
Prof Maxence Bigerelle
LAMIH UMR UPHF/CNRS 8201
+33 (0)3 89 60 87 66 (Anselme)
+33 (0)3 44 23 46 17 (Bigerelle)
karine.anselme@uha.fr
Maxence.Bigerelle@uphf.fr
www.is2m.uha.fr
www.uphf.fr/LAMIH/
Please note, this article will also appear in the first edition of our new quarterly publication. Subscribe to our updates for free here.