The Laboratoire Radiopharmaceutiques Biocliniques has a high-level expertise in the development of novel radioactive compounds for the diagnosis and therapy of diseases representing major public health issues.
Nuclear medicine is based on the administration of radiopharmaceuticals, the specialism of Laboratoire Radiopharmaceutiques Biocliniques, to patients for the diagnosis, monitoring, and treatment of diseases.
What are radiopharmaceuticals?
Radiopharmaceuticals are compounds labelled with radioactive isotopes and specific for a molecular target or a pathophysiological process. Radioactive isotopes used for labelling might be chosen either for diagnostic imaging or radiotherapy.
The objective of Laboratory Radiopharmaceutiques Biocliniques, Grenoble, France, is to develop new radiopharmaceuticals for solving commonly encountered clinical problems in the fields of cardiology, oncology, and metabolic and neurodegenerative diseases. Such a development is initiated through discussions with physicians in order to precisely define the clinical problem to be solved, and is completed when the pre-clinically selected compound undergoes clinical validation through Phase I, II, and III clinical trials.
The successful fulfilment of this objective by Laboratoire Radiopharmaceutiques Biocliniques is ensured by the multidisciplinary skills of its members from radiochemistry to biology and clinical practice, as well as state-of-the-art preclinical and clinical equipment (France Life Imaging network).
The following paragraphs outline two selected examples of original radiopharmaceuticals currently under development in the Laboratoire Radiopharmaceutiques Biocliniques in the fields of cardiology and oncology.
Cardiology – vulnerable plaque imaging
According to the Global Burden of Disease study, with 17.6 million deaths each year, cardiovascular diseases are the leading cause of mortality worldwide, with most of these deaths being due to coronary artery disease (CAD). Although general risk factors allowing initial population risk stratification are well known, the identification of high-risk individuals before the occurrence of an acute coronary event remains a major challenge for the efficient prevention of potentially fatal coronary events.
The underlying mechanism of CAD is the development of atherosclerotic plaques within the walls of coronary arteries. Some of these plaques, described as ‘vulnerable atherosclerotic plaques’, initially develop eccentrically without affecting the intraluminal diameter of coronary arteries and correspond to an inflammatory process of the vessel wall.
Accordingly, two-thirds of coronary events are caused by the rupture of an atherosclerotic lesion, inducing a <50% coronary stenosis. As intravascular coronarography only assesses vessel lumen, this widely used invasive methodology is not suitable for the identification of most vulnerable atherosclerotic lesions. There is, therefore, a strong need for a non-invasive diagnostic tool allowing the identification of vulnerable lesions in clinical practice
The nuclear imaging of coronary inflammatory vulnerable plaques remains a challenge, mostly because of the small volume of the lesions and their vicinity with the blood containing an unbound circulatory tracer which affects image contrast. Thus, an ideal imaging agent should combine high affinity and specificity, good solubility and stability, and efficient radiolabeling with a fast blood clearance so that high-contrast images can be obtained shortly after administration.
Single domain antibodies
Due to their small size, single domain antibodies (sdAbs), which are composed of the single variable domain of the heavy chain of antibodies, constitute a promising new class of radiotracers that fulfills these requirements. Therefore, for the purpose of vulnerable lesion imaging, sdAb-based radiotracers directed against the Vascular Cell Adhesion Molecule 1 (VCAM-1), an adhesion molecule that is expressed during the inflammatory processes in vulnerable lesions but not in healthy arteries, were produced and evaluated by the Laboratoire Radiopharmaceutiques Biocliniques.
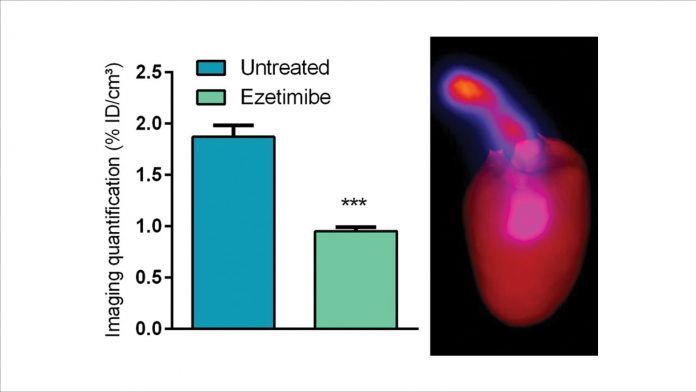
Evaluations were performed in vitro on mouse and human recombinant VCAM-1 proteins and endothelial cell-cultures, and in vivo in an Apolipoprotein-E deficient mouse model of atherosclerosis (ApoE-/-). Among ten evaluated sdAbs, the lead compound, 99mTcAbVCAM1-5, exhibited exquisite properties for the non-invasive molecular imaging of atherosclerotic lesions. Indeed, murine aortic plaques were successfully visualised noninvasively by nuclear imaging. Furthermore, the sensitivity of 99mTc-cAbVCAM1-5 imaging was successfully demonstrated using gold standard therapies (Statin, ezetimibe) (see Fig. 1).
Based on these encouraging preclinical findings, 99mTcAbVCAM1-5 is currently undergoing clinical translation. The production of cAbVCAM1-5 has been performed according to Good Manufacturing Practices (GMP), and a first in human clinical study in healthy volunteers and patients will now be conducted in collaboration with the Cardiology Department of Grenoble-Alpes University Hospital, France. The primary end point of this phase I/IIa trial will be the evaluation of the safety of this new imaging agent, as well as its ability to bind to VCAM-1-positive lesions. Results from this clinical study are expected in 2019.
Oncology – new theranostic agents
Cancer remains another leading cause of human death, despite intense clinical research efforts. Challenges in oncology include the fact that conventional therapies are not always efficient and are responsible for severe side effects. New therapies are therefore being developed in order to overcome these limitations, directed towards various targets specifically expressed by tumor subsets.
In this setting, new agents are needed that will allow the imaging of primary tumour and metastasis phenotypes in order to identify those patients who will benefit the most from such specific treatments. These radiopharmaceuticals which allow personalised prediction and follow up of the effect of a specific therapy have been referred to as ‘companion markers’.
In the field of nuclear medicine, radiopharmaceuticals are therefore developed for use as companion markers of specific immunotherapies. The theranostic strategy is another example in which the development of highly specific imaging agents is required. Here, the coupling between diagnostic imaging and therapy is performed using the same molecule, radiolabeled with a radioelement dedicated either to imaging for a diagnostic purpose or to radiotherapy for a therapeutic purpose. One of the aims of the Laboratoire Radiopharmaceutiques Biocliniques is to develop such companions and new theranostic agents consistently with the rationale of ‘4P Medicine’ (personalised, predictive, preventive, participatory).
RAFT-RGD
Integrins are membrane proteins which are overexpressed in several aggressive cancers. A promising theranostic agent targeting the αvβ3 integrin has recently been developed in the laboratory. The ligand is a small molecule called RAFT-RGD. The RAFT-RGD has been successfully radiolabelled with 99mTc or 68Ga for the diagnosis and with 90Y or 177Lu for therapy. Pre-clinical studies have demonstrated the theranostic potential of this agent. A human clinical study will be conducted in patients with metastatic breast cancer and glioblastoma at the Grenoble-Alpes University Hospital. The safety, as well as the ability to bind αvβ3 lesions, will be evaluated in a phase I/IIa trial in 2019.
NeoBOMB
In parallel, a collaboration has been established between Advanced Accelerator Application (AAA), a Novartis company, and the laboratory for the pre-clinical and clinical evaluation of theranostic agents. Recently, a gastrin-releasing peptide receptor targeting agents that can either be labeled with 68Ga for diagnosis (68Ga-NeoBOMB1) or 177Lu for therapy (177Lu-NeoBOMB1) has been validated on a murine model (see Fig. 2). A phase II multi-centre clinical trial co-ordinated at the national level by Laboratoire Radiopharmaceutiques Biocliniques/Grenoble-Alpes University Hospital is currently in progress in order to evaluate the potential of this new theranostic agent.
Conclusions
The development of radiopharmaceuticals is submitted to standard regulatory requirements for drug development and clinical research. Each step of this process requires advanced scientific skills, up-to-date equipment and significant financial support. In addition, partnership between academic laboratories and pharmaceutical companies is mandatory for efficiently conducting phase II and III clinical trials.
Current strategies for the development of new radiopharmaceuticals have the potential to address major human health issues. Thanks to its long established experience in translational research, Laboratoire Radiopharmaceutiques Biocliniques built a unique chain of expertise for the development of these new radiopharmaceuticals.
Once translated into the clinical arena, these novel agents will strengthen the role of nuclear medicine as a powerful tool for 4P medicine.
Professor Catherine GHEZZI, PhD
Laboratoire Radiopharmaceutiques Biocliniques
INSERM – Université Grenoble-Alpes
+33(0)4 76 63 74 76