Professor Conchi O Ania, Research Director of CNRS at CEMHTI, discusses the possibilities of metal-free porous carbons in energy and environmental applications.
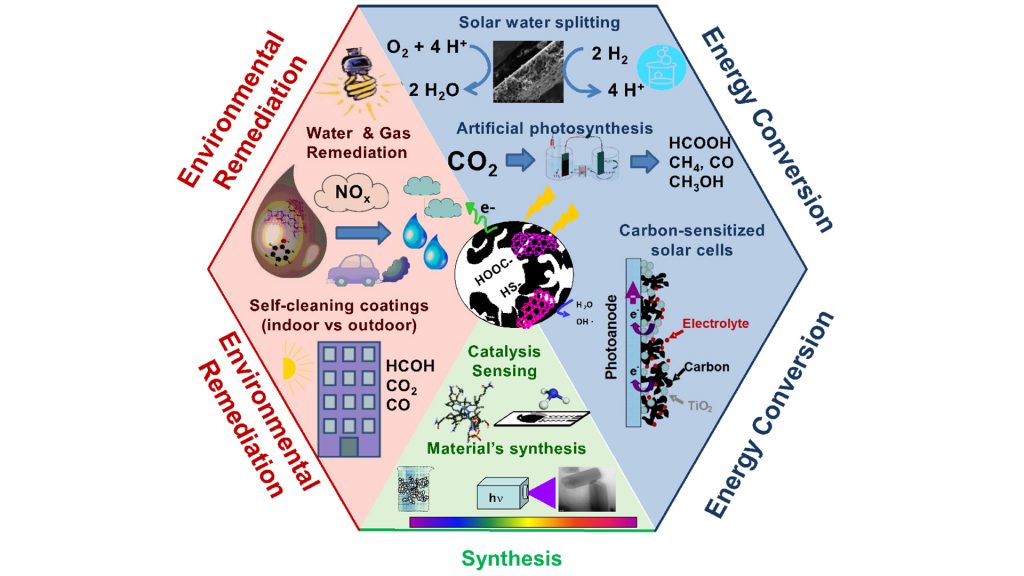
Metal-based catalysis is ruling the world’s most important industrial processes, including energy generation, conversion and storage. However, metal-based catalysis faces multiple disadvantages related to the high cost of precious metals (very often most active metal-based catalysts), low durability due to poisoning during the reaction operation, limited and uneven geographical distribution of the reserves of precious metals, and low sustainability. Overcoming such drawbacks with metal-free materials based on abundant renewable resources has become a priority of most research programmes, aiming to boost the large-scale commercial applications of sustainable and renewable energy generation/conversion/storage technologies (e.g., fuel cells, batteries, supercapacitors, water splitting, carbon dioxide conversion into fuels, nitrogen fixation), as promising solutions towards current energetic and environmental scenarios.
The proof-of-concept of the potentialities of metal-free carbon-based materials as alternatives to metallic catalysts was first demonstrated in 2009 for the electrochemical reduction of oxygen (Science 2009, 323, 760). Since then, many different forms of carbon (graphene, carbon nanotubes, carbon nitride, porous carbons) or their combinations have demonstrated high catalytic activities (with better performance and durability for certain carbons) than state-of-the-art, non-precious metal catalysts for energy conversion/storage and environmental protection. These findings have garnered the interest on the use of carbon-based catalysts. Indeed, owing to the versatility and availability of carbons with varied morphology, composition, and pore architecture, as well as the abundance of precursors, it is highly feasible to push the use of carbons as promising sustainable alternatives to metallic catalysts.
Porous carbons
Among the variety of carbon materials, porous carbons – also known as activated carbons – have attracted increasing attention in the last decades in the field of catalysis. Porous carbons are some of the oldest materials known to human beings, with a long history of applications going back to BC times (metal age, inks in cave paintings, Chinese ink, water purification, etc.). Their fabrication has ancient origins, and current manufacturing processes have been known for more than a century. Due to their low cost and large-scale production, porous carbons are essential materials in the technological applications of multidisciplinary fields, although they have been traditionally applied as adsorbents in liquid and gas purification processes, where they have almost no competitors.
The discovery of graphene (and its derivatives) and the poor performance of defect-free graphene sheets in numerous applications – including catalysis – motivated the interest for porous carbons from a new perspective in certain catalytic domains. Indeed, the reactivity of the highly defective graphenic layers present in the pores of disordered porous carbons has been revealed to be the key towards novel applications. Other advantages of porous carbons are their porosity, surface chemistry, and a good level of conductivity – even if lower than in graphite or graphene – which can be modulated to fulfil the requirements of a specific application.
The combination of all these features is unique to porous carbons, and a powerful advantage of the capabilities of porous carbons in new fields of application. The challenge lies in rationalising their behaviour in cutting edge applications related to energy storage/conversion, environmental remediation, catalysis, etc.
Solar renewable energy
In a world that urgently needs to redress the balance of energy derived from fossil fuels, the efficient conversion of sunlight into chemical energy is the key towards combating climate change, environmental pollution, and natural resource challenges. With solar energy being the primary and practical source of energy in the world, it should take a step forward in the energetic scenario in the near future to provide the clean, affordable, multi-source and multi-purpose fuels needed to achieve the intertwined goals of economic growth, environmental conservation, and energy security (the so-called ‘3 Es’), which are the pillars of green development.
Artificial photosynthesis
Contrary to solar electricity, solar fuels have the gigaton-scalability and multi-functionality to substitute coal and oil in major energy sectors (industry, transportation, and petrochemicals), accomplishing the transition to a future powered by sunlight. Among the different options to convert solar energy into easily storable chemicals (solar fuels), artificial photosynthesis – solar-powered water splitting to produce hydrogen/oxygen, and the solar-driven photo-reforming of carbon dioxide – appears to be a key solution. However, this process is technically complex and a number of scientific and technological challenges remain to be solved in order to successfully scale-up existing lab-prototypes towards commercialisation. The ideal concept of a direct conversion of light into chemical energy has several limitations, and practical applications lie at the crossroads of basic physics and the chemistry of materials and energy technology. Materials must efficiently harvest sunlight in natural environments, and the majority of systems for solar fuels production use metal oxides and noble metal catalysts (which are scarce, expensive and unsustainable).
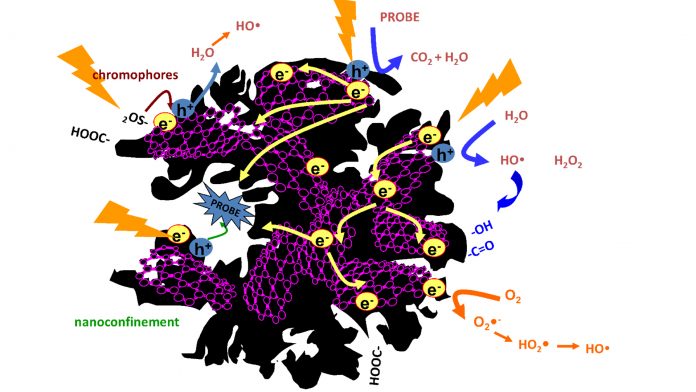
Recent studies have pointed out the potential of certain carbons to provide an affordable alternative to replace metallic catalysts for the production of solar fuels, overcoming technical challenges, complying with sustainability, and capable of bringing down costs to competitive levels. Carbon catalysts are capable of promoting the in-situ reduction of carbon dioxide and the oxidation of water to produce energy carriers (i.e., from C1 – methane, formaldehyde, and methanol – to C2-C4 fuels) through appropriate modifications (hole/electron donor doping, chromophore sites, porosity) to tailor the catalytic centres. Despite such promising results, the area is still in its infancy.
Owing to the complexity of carbon catalysts, the exploitation of such abilities for the production of solar fuels is not straightforward and much work needs to be done in the appropriate direction. The optimisation of carbon catalysts and interfaces can be performed by creating separate sites for oxidation and reduction reactions, while the selectivity can be controlled by tailoring the nanopore confinement and hydrophobic nature to commensurate redox potentials and affinity of the target molecules.
An adequate choice of the carbon characteristics may render materials with a high light harvesting ability that would overcome the low photonic yield of certain semiconductors under sunlight, as well as increasing the overall efficiency. As photocatalysts, porous carbons also offer the advantage of coupling their high adsorption capability to a tuneable photoactivity; the challenge is in further enhancing the photochemical activity by balancing the surface composition, porosity, and change-carrier mobility.
Photochemistry based on nanoporous carbons: an ERC Consolidator project
Nanoporous carbons are capable of efficiently absorbing photons, which can then be converted into photochemical reactions in confined nanopore spaces. This is the core of the PHOROSOL project, an ERC-backed initiative [grant agreement No. 648161] investigating the potential use of light responsive nanoporous carbons in interdisciplinary fields of applied photochemistry for gas sensing, energy conversion, and environmental protection. The ambition of the project is to profit from the dual nature of nanoporous carbons as strong light absorbing materials with unique electronic features and nanoporosity in order to boost technologies for light conversion where a fast, sensitive, and selective response is needed.
In 2010, our group demonstrated the photochemical activity of a semiconductor-free nanoporous carbon towards the degradation of phenol in solution (Appl. Surf. Sci., 256, 2010, 5254). Since then, and aiming at understanding this behaviour and the mechanisms governing the photochemical activity of carbons, we have explored a variety of carbon materials with different textural properties and composition. A better understanding of the nature of the light/carbon interactions can lead to a full exploitation of their potential in several photochemical reactions. Evaluating the performance of these materials using standard methods is complex due to the challenges associated with discriminating the photons absorbed into the carbon matrix itself from the fraction of light capable of being converted into chemical reactions. This is a central part of the PHOROSOL research agenda, along with modifying and tailoring the properties of these materials. By building a deeper understanding of the origin of the photoactivity of porous carbons, we hope to lay the foundations for boosting their integration in a plethora of novel applications.
Several research directions are being explored:
Porous carbons as metal-free, semiconductor-free photocatalysts
Following our pioneering studies demonstrating the photochemical activity of porous carbons, our research has been focused on understanding the mechanisms governing the carbon photoinduced reactions and the fate of the photoexcited states of the carbon atoms. Data has demonstrated the correlation of the photochemical activity of carbons with the formation of radical species in the presence of oxygen and water and upon irradiation in the full UV-VIS spectrum. The formation photogenerated charge carriers (electrons and holes) upon irradiation have been confirmed, as well as their stabilisation through chemical reactions with other species confined in the pores (Adv. Sci. 5, 2018, 1800293).
Understanding the photochemical activity of different carbon materials has opened up new perspectives in the field of metal-free photoactive materials for energy conversion, environmental remediation and sensing applications. For instance, the application of hybrid technological solutions coupling adsorption on porous carbons with advanced oxidation processes based on photocatalysis is an interesting solution in the field of wastewater remediation (J. Colloids Interf. Sci. 490, 2017, 879). Such hybrid systems allow high conversion efficiencies to be achieved (in terms of pollutant degradation) and the photoinduced reactivation of the exhausted carbon to be promoted, thereby extending its lifetime and lowering the reactivation cost – enabling on-site regeneration.
Porous carbon catalysts for solar energy conversion
Following our results on the photochemical activity of nanoporous carbons towards the oxidation of pollutants and their ability to generate radical species in water, we decided to explore the application of photoresponsive porous carbons in transforming CO2 and H2O into sustainable fuels, which is now a major global research priority. As photoanodes for the photo-reforming of water, remarkable photocurrents have been recorded for carbons with varied compositions and at overpotentials lower than those reported for benchmark metal-catalysts. Even though the photocurrent values are not as high as those reported for other water splitting photocatalysts, they are outstanding for metal-free photoanodes. The surface functionalisation of the carbon (S-, N-, O- groups) has revealed to be an important factor for its performance as photoanode. Photocurrent densities can be increased in functionalised materials provided that the adequate moieties are created and that the conductivity of the anodes is not compromised (Molecules 21, 2016, 1611).
A balance of both aspects is essential to prevent the photo-corrosion of the anodes upon long-term operation, giving rise to deactivation of the photoactive sites and to charge transfer limitations. In the photo-reduction of carbon dioxide, we have obtained some promising results combining functionality and porosity of carbons towards the conversion into carbon monoxide in dry conditions. We are now looking at how this can be optimised to synthesise long fuel molecules.
Porous carbons as additives to semiconductors in photocatalytic paints
The use of carbon materials as additives to semiconductors has been a widely investigated area in the last decade, aiming to overcome the low photonic efficiency of most semiconductor photocatalysts under sunlight. Amongst others, various forms of carbons have proven to be efficient additives for inducing an efficient charge separation and reducing surface recombination, with most research directed towards applications in solution. Based on the observed intrinsic photochemical activity of certain porous carbons, we have explored their use as additives in the formulation of self-cleaning photocatalytic paints with activity under sunlight and their performance for the degradation of environmental pollutants in gas phase (with applications in the purification of indoor and outdoor environments).
Photoactive carbons as additives enhance the visible light harvesting ability of the semiconductor and favour the charge transfer at the carbon/semiconductor interface and inside the nanopores. As a result, the photocatalytic paints are able to degrade the adsorbed pollutants and their decomposition intermediates in dry conditions, showing good cyclic performance and stability during long-term illumination. The role of the pollutants’ nanoconfinement and their interfacial interactions with the pore walls of the carbon additives seem to be important factors that control the overall photoconversion yields.
These hybrid semiconductor/carbon materials have also been used in photoelectrochemical water oxidation, where the carbon additive is an interesting strategy for improving the photoelectrochemical performance of nanostructured semiconductors featuring hindered carrier transport (C-journal 4, 2018, 45). The incorporation of increasing amounts of porous carbons of low functionalisation to semiconductors have improved the quantum yield of the reaction. The carbon matrix affects the dynamics of the charge transport, creating a percolation path for the majority carriers which are easily delocalised through the graphitic layers.
Photo-assisted synthesis of porous carbons
The design of porous carbons with precise control of the pores structure and chemical composition is a widely investigated topic, driven by the need of selective and efficient carbons in a variety of application fields. In view of this, we are investigating the use of light activation to promote the cross-linking of polymer precursors (Carbon 116, 2017, 264) in the synthesis of porous carbons with controlled features. This synthetic route under controlled illumination conditions offers several advantages for the preparation of porous carbons over conventional methods, such as much shorter time scales – ageing time needed to induce the cross-linking of the polymeric precursor is significantly reduced – and the versatility of organic molecules as precursors. We have demonstrated that by changing the chemistry of the organic precursor it is possible to control the porosity of the obtained carbons, as it is defined by the structure of the precursor and the number of reactive positions. For instance, the photopolymerisation of linear dihydroxylated precursors gives rise to dense porous carbons mainly composed of narrow micropores, whereas trihydroxylated molecules render carbons with wider pore size distributions in the full nanometric scale.
Implications of nanopore confinement at extreme conditions
A tight nanopore confinement of certain probes – oxidisers – in porous carbons has been revealed to be critical to the performance of the resulting material in terms of releasing thermal energy (patent PAT-10381-BE01, Belgium; Carbon , 2020, 129). Some mixtures are capable of competing with the best performing nanoenergetic materials in the market for civil and military applications, with better safety performance. While a great deal of progress has been made in this respect over the course of the project so far, there is still significant scope to further improve efficiency. We are now working to control the stability in different environments.
The photoluminescence of porous carbons
Addressing the luminescence characteristics of carbon materials is a challenging task due to the strong light absorption of the carbon matrix itself. As a result, the phenomenon of photoluminescence has only been demonstrated for certain members of the carbon family with high electron mobility and a high degree of transparency, namely graphene-derivatives, carbon nanotubes, and carbon quantum dots.
We have recently been able to measure light emission features in some porous carbons, which could have a significant impact on the development of sensors with improved sensitivity and selectivity. We are now working to control the emission/absorption wavelength. We have observed that the polarity of the solvent and the temperature of the analysis greatly influence the light emission spectra of the studied carbons, otherwise characterised by a weak intensity. Furthermore, the formation of short and long-lived transient species in the nanosecond scale, attributed to various excited states, has been confirmed for porous carbons.

References
Ania CO, Armstrong P, Bandosz TJ, Beguin F, Carvalho AP, Celzard A, Frackowiak E, Gilarranz MA, Laszlo K, Matos J, Pereira MFP. ‘Engaging Nanoporous Carbons in ‘Beyond Adsorption’ applications: Characterization Challenges and Performance’, Carbon 164 (2020) 69
Balan L, Fernandez de Cordoba MC, Zaier M, Ania CO, ‘A green and fast approach to nanoporous carbons with tuned porosity: UV-assisted condensation of organic compounds at room temperature’, Carbon 116 (2017) 264
Bandosz TJ, Ania CO, ‘Origin and Perspectives of the Photochemical Activity of Nanoporous Carbons’, Adv. Sci. 5 (2018) 1800293
Gesesse GD, Gomis-Berenguer A, Barthe MF, Ania CO, ‘On the analysis of diffuse reflectance measurements to estimate the optical properties of amorphous porous carbons and semiconductor/carbon catalysts,’ J. Photochem. Photobiol. A Chem. 398 (2020) 112622
Gomis-Berenguer A, Eliani I, Lourenço VF, Carmona RJ, Velasco LF, Ania CO, ‘Insights on the use of carbon additives as promoters of the visible-light photocatalytic activity of Bi2WO6’, Materials 12 (2019) 385
Gomis-Berenguer A, Velasco LF, Velo-Gala I, Ania CO, ‘Photochemistry based on nanoporous carbons: perspectives in energy conversion and environmental remediation’,. Colloid Interf. Sci. 490 (2017) 879-901
Gomis-Berenguer A, Velo-Gala I, Rodriguez-Castellón E, Ania CO, ‘Surface Modification of a Nanoporous Carbon Photoanode upon Irradiation’, Molecules 21 (2016) 1611
Gomis-Berenguer A, Celorrio V, Iniesta J, Fermin DJ, Ania CO, ‘Nanoporous carbon/WO3 anodes for an enhanced water photooxidation’, Carbon 108 (2016) 471
Gomis-Berenguer A, Iniesta J, Maurino V, Lima JC, Ania CO, ‘Nanoconfinement and wavelength dependence of the photochemistry of nanoporous carbons’, Carbon 96 (2016) 98
Quezada A, Ruiz-Garcia C, Sauvage T, Chazaro LF, Rangel R, Ania CO, ‘Photochemical and electrochemical reduction of GO thin films: tuning the nature of surface defects’, Phys. Chem. Chem. Phys. 22 (2020) 20732
Van Riet R, Amayuelas E, Lodewyckx P, Lefebfre MH, Ania CO, ‘Novel Opportunities for Nanoporous Carbons as Energetic Materials’, Carbon 164 (2020) 129
Please note, this article will also appear in the fifth edition of our quarterly publication.