Katja Heinze, from the Johannes Gutenberg University Mainz, Germany, explains a successful approach for the design of sustainable near-infrared emissive materials with earth-abundant elements.
Red and near-infrared luminescence (NIR: wavelengths above 750 nanometres) in molecular complexes with metal ions has matured into a well-developed and thoroughly investigated research field with a plethora of important photonic applications in various fields, including lasers, bioimaging, (time-resolved) immuno assays, security inks for banknotes (e.g. euro banknotes) or optical telecommunication. However, the desired effect of luminescence in the NIR with reasonable quantum yields is typically achieved using rare earth metal ions or noble metal ions. Both the rare earth elements and the noble metals are critical raw materials and consequently any substitute with earth-abundant metal ions is extremely welcome.
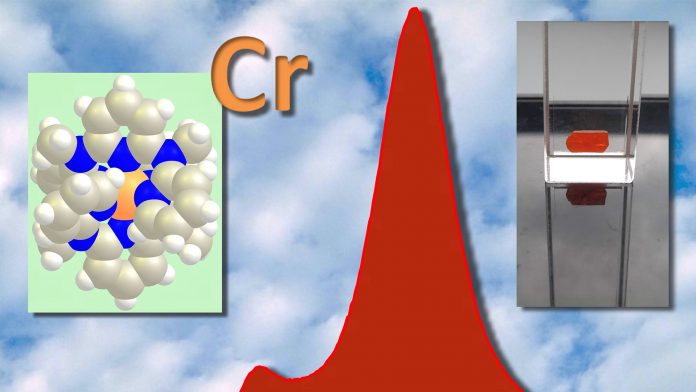
Until very recently, essentially no potent substitutes using earth-abundant metal ions had been developed. The typically poor performance in luminescence, especially NIR luminescence, is an intrinsic feature of the excited state ordering of first row transition metal complexes (scandium to zinc) which constitute the majority of the metals on earth. To overcome this deficiency, we have developed a suitable environment, the ligand, for the transition metal ion.1 This measure has been very successful using chromium as central metal ion. The natural gemstone ruby, which displays similar features of luminescence, has inspired the underling conceptual idea. In contrast to the insoluble solid state material ruby, the newly developed chromium complex is a soluble molecule amenable to further modification and applications in solution, including biological media. Based on the very high quantum yield and excited state lifetime achieved with the chromium complex and its molecular nature, this material is now coined ‘molecular ruby’.
First applications of the luminescent ‘molecular ruby’ with the long lifetime were already demonstrated together with collaboration partners from the Bundesanstalt für Materialforschung Berlin (Dr Ute Resch-Genger), the University of Montréal (Professor Christian Reber) and the University Mainz (Professor Till Opatz).2-4
Unique applications
These applications include optical temperature sensing in solution, in micelles and in nanoparticles, optical pressure sensing in solution and in the solid state and photocatalysis using oxygen as terminal oxidant. These unique applications were enabled based on the unprecedented high emission quantum yield above 10% even in solution and the very long lifetime of almost a millisecond. Together with Professor Michael Seitz from the University of Tübingen, our group could enhance these already exceptionally favorable optical properties by optimising the environment of the chromium ions, especially by replacing hydrogen atoms by deuterium atoms at decisive positions in the ligand and in the solvent.5 This modification boosted the quantum yield and lifetime to record values of 30% and to over two milliseconds, respectively. Many more applications of the ‘molecular ruby’ are within reach.
Other earth abundant metal ions
With this first successful molecular material based on earth-abundant elements in hand and an increasingly deeper photophysical understanding, we are now in process of expanding this research direction to other earth abundant metal ions. These fundamental developments are advanced within the Priority Program 2102 ‘Light controlled reactivity of metal complexes’ of the German Research Foundation, co-ordinated by Katja Heinze and bringing together more than 15 researchers from Germany, Switzerland and Austria aiming at a deeper understanding of photophysical and photochemical processes in transition metal complexes.
References
- S. Otto, M. Grabolle, C. Förster, C. Kreitner, U. Resch-Genger, K. Heinze, Angew. Chem. Int. Ed. 2015, 54, 11572-11576
- S. Otto, N. Scholz, T. Behnke, U. Resch-Genger, K. Heinze, Chem. Eur. J. 2017, 23, 12131-12135
- S. Otto, A. M. Nauth, E. Ermilov, N. Scholz, A. Friedrich, U. Resch-Genger, S. Lochbrunner, T. Opatz, K. Heinze, ChemPhotoChem 2017, 1, 344
- S. Otto, J. Harris, K. Heinze, C. Reber, Angew. Chem. Int Ed. 2018, 57, in press (DOI: 10.1002/10.1002/anie.201806755)
- C. Wang, S. Otto, M. Dorn, E. Kreidt, J. Lebon, L. Sršan, P. Di Martino-Fumo, M. Gerhards, U. Resch-Genger, M. Seitz, K. Heinze, Angew. Chem. Int. Ed. 2018, 57, 1112-1116
Professor Katja Heinze
Chair of Inorganic Chemistry
Johannes Gutenberg University
Institute of Inorganic Chemistry and Analytical Chemistry
katja.heinze@uni-mainz.de