Led by Professor Nicola Forgione, the Nuclear Research Team at the University of Pisa is aiming to improve the understanding of lithium-water interactions to improve tokamak reactor safety.
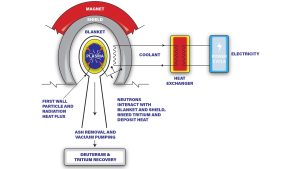
Magnetic confinement fusion reactors have the potential to be a sustainable and almost infinite source of energy. Fusion reactors, unlike conventional nuclear reactors, are trying to duplicate the processes that take place in the sun. Hydrogen nuclei fuse to form helium, releasing huge amounts of energy. The Breeding Blanket, one of the main components of any fusion reactor, serves the purpose of generating (or ‘breeding’) further tritium fuel (which is required for the nuclear fusion reaction) and collecting the thermal energy generated by the plasma, as shown in Fig. 1.
Candidates for Breeding Blanket
Four different concepts are developed as candidates for Breeding Blanket, each with unique advantages and challenges.
Water Cooled Lead Lithium (WCLL)
The WCLL system¹ uses water as a coolant, operating under conditions similar to those of Pressurised Water Reactors (pressure of 15.5 MPa and temperature from 295 to 328°C). The PbLi eutectic serves as the breeder material, and tritium is extracted from the PbLi outside the reactor.
The WCLL system offers two main advantages that make it a promising solution for implementation in magnetic confinement fusion reactors:
- Compatibility with existing nuclear technology: This choice ensures compatibility with existing nuclear technology and infrastructure, operating under conditions similar to those of PWRs.
- Safety and Tritium Management: The PbLi eutectic used as the breeder material in the WCLL system enables efficient tritium extraction outside the reactor. This approach enhances operational safety, as tritium, a radioactive isotope, can be managed and contained more safely outside the reactor.
Helium Cooled Pebble Bed (HCPB)
The HCPB system² uses helium as a coolant (pressure of 8 MPa and temperature from 300 to 500°C), which has the advantage of being an inert gas and having relatively high thermal conductivity. The use of Li4SiO4 or Li2TiO3 as breeder materials, along with beryllium, helps in efficient tritium breeding and heat transfer. The tritium extraction occurs within the blanket using a purge gas, which simplifies the design and operation.
Helium Cooled Lead Lithium (HCLL)
The HCLL system³ also uses helium as a coolant (at a pressure of 8 MPa and temperature from 300-500°C) but employs a PbLi eutectic as the breeder material. This combination allows for efficient heat transfer and tritium breeding. The tritium is extracted from the PbLi outside the reactor, which can be advantageous for maintenance and safety.
Dual Coolant Lead Lithium (DCLL)
The DCLL system⁴ uses a dual coolant approach, with helium cooling the first wall at a pressure of 8 MPa and temperature range of 300-400°C, and PbLi serving simultaneously as a coolant and breeder, with a maximum operational temperature of 500°C. This design allows for higher operational temperatures and efficient heat transfer. The tritium extraction system is located outside the reactor, which can enhance safety and ease of maintenance.
The role of the Nuclear Research Team at the University of Pisa
The Nuclear Research Team led by Prof Nicola Forgione at the Department of Civil and Industrial Engineering of the University of Pisa (DICI-UNIPI) is actively involved in this technology, focusing on the safety aspects of the WCLL concept, particularly for the analysis of a hypothetical In-Box Loss of Coolant Accidents (LOCAs) involving lithium-water interactions.5-7
The chemical reaction between lithium contained inside the Li-Pb alloy and water is an important phenomenon which significantly influences the behaviour of the overall interaction; it can be done in two different ways:
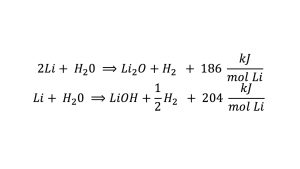
As can be seen, these reactions are highly exothermic, leading to rapid temperature increases and the formation of hydrogen gas. Therefore, the primary objective of the research activities being carried out at DICI-UNIPI is to enhance the validation of numerical codes to be used for safety analyses of magnetic confinement fusion reactors by improving the understanding of the transient phenomena that occur during LOCAs, with interaction leading to complex chemical reactions and thermal dynamics that can pose safety consequences.
Our research employs computational approaches to account for lithium-water interactions in a specific way, supported by available experimental data. In our Nuclear Thermal-Hydraulic Simulation Laboratory, we modelled LOCAs by considering controlled amounts of water in a lithium-lead environment, considering the same operating conditions foreseen for the fusion reactor.
In particular, we have developed calculation models implemented in numerical codes capable of predicting the behaviour of lithium-water interactions in various scenarios. These models have been validated against experimental data available in the literature to ensure accuracy and reliability.
In conclusion, the research conducted by our team at DICI-UNIPI represents a step forward in the development of safe and efficient magnetic fusion reactors. Understanding and mitigating the risks associated with lithium-water interactions in the case of LOCAs, we contribute to making fusion energy a credible and sustainable future-proof energy source.
References
- Del Nevo, A. et al. (2017). WCLL breeding blanket design and integration for DEMO 2015: Status and perspectives. Fusion Engineering and Design, Vol. 124, pp. 682–686.
- Hernández, F.A. et al. (2020). Consolidated Design of the HCPB Breeding Blanket for the Pre-Conceptual Design Phase of the EU DEMO and Harmonization with the ITER HCPB TBM Program. Fusion Engineering and Design, Vol. 157, 111614.
- Aiello, G., et al. (2014). Development of the Helium Cooled Lithium Lead blanket for DEMO. Fusion Engineering and Design, Vol. 89, pp. 1444-1450.
- Rapisarda, D. et al. (2016). Conceptual Design of the EU-DEMO Dual Coolant Lithium Lead Equatorial Module, IEEE Transactions on Plasma Science, Vol. 44, pp. 1603-1612.
- Galleni, F. et al. (2021). Numerical simulation of an out-vessel loss of coolant from the breeder primary loop due to a large rupture of tubes in a primary heat exchanger in the DEMO WCLL concept. Energies, Vol. 14 (21), art. no. 6916.
- Galleni F. et al. (2023). Development of a coupling technique between RELAP5 and SIMMER-IV for fusion reactor applications. Fusion Engineering and Design, Vol.193, art. no. 113682.
- Cossu, V. et al. (2024), Application of a multiphysics simulation tool to an in-box LOCA in the WCLL TBM test section of the LIFUS5/Mod4 facility. Fusion Engineering and Design, Vol. 200, 114219.
Please note, this article will also appear in the 21st edition of our quarterly publication.