Gareth Bolitho, Commercial Director of Cellexus, discusses the potential of phage therapy in combatting the rising global threat of antimicrobial resistance.
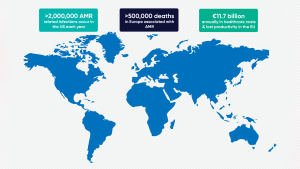
Antimicrobial resistance (AMR) stands as one of the most urgent global health threats of our time, undermining decades of progress in medicine, agriculture, and public health. According to the World Health Organization (WHO), over 500,000 deaths in Europe alone are linked to drug-resistant bacterial infections each year – a burden equal to influenza, tuberculosis, and HIV/AIDS combined. The economic toll is truly staggering, with the European Centre for Disease Prevention and Control (ECDC) estimating €11.7bn annually in healthcare costs and lost productivity across the EU.¹
As bacteria continue to outpace the efficacy of conventional antibiotics, the search for innovative alternatives has never been more critical. Bacteriophage therapy, a century-old concept, is currently being revitalised. Modern science is enabling a deeper understanding of bacteriophages and their potential applications, leading to increased research, clinical trials, and even compassionate use cases, presenting phage therapy as a promising solution that offers targeted, sustainable approaches to combat resistant infections across healthcare, biotechnology, and agriculture.1,2
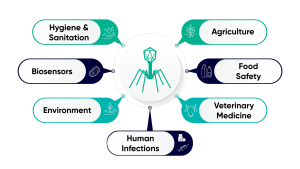
Phage therapy: A precision tool against AMR
Unlike broad-spectrum antibiotics, bacteriophages (phages) are viruses that specifically infect and lyse bacteria, leaving the beneficial human microbiota unharmed. This specificity reduces selective pressure on commensal bacteria, lowers the risk of resistance spreading, and minimises collateral damage to the microbiome.¹ Clinical successes have been reported for unmanageable infections, including cases where conventional antibiotic treatments failed, such as in the case of methicillin-resistant Staphylococcus aureus (MRSA).2,3
Growing interest in phage therapy extends beyond human health. In veterinary medicine, phages are deployed to treat infections in livestock and companion animals, offering an alternative to antibiotics and contributing to lower AMR rates in the food chain.⁴ In agriculture, phage-based treatments are used to manage bacterial diseases in crops like tomatoes, peppers, and potatoes, offering an eco-friendly, targeted alternative to chemical pesticides and antibiotics.⁵ Meanwhile, in aquaculture, phages are increasingly explored to manage bacterial outbreaks in fish farming, and to reduce contamination in water systems.
From innovation to scale: The gap
Despite this promising momentum, a significant gap remains between laboratory innovation and real-world impact. Scaling phage therapy for widespread clinical and commercial use is still constrained by limited manufacturing capacity and evolving regulatory frameworks. While researchers have produced thousands of phage strains and demonstrated efficacy in both clinical trials and compassionate-use settings, the infrastructure to manufacture, purify, and deliver phage products at scale remains limited.1,2 Most countries currently lack the specialised facilities required for Good Manufacturing Practice (GMP) phage production.1,6 The UK, for example, has no national GMP phage manufacturing capacity, posing a significant barrier to clinical adoption.⁷
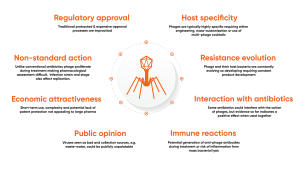
Another key barrier is the cost and complexity of adapting conventional bioreactors for phage production. Implementing facilities to clean and sterilise stainless-steel or single-use systems is both highly expensive and requires extensive ongoing supervision and validation. These processes increase manufacturing time and cost, creating a massive challenge for efficiently scaling production.
What is needed in today’s world?
Achieving scalable phage therapy will demand a multifaceted approach. Investing in dedicated GMP- compliant manufacturing infrastructure is essential. Facilities built for purpose are needed to support both off-the-shelf phage cocktails and personalised therapies. This will help to enable local manufacturing, and accelerate clinical translation.⁷ This is where Cellexus offers a clear advantage. The company’s CellMaker is an advanced single-use airlift bioreactor, proven to be highly effective for phage production. It provides a closed-loop system that eliminates the time, capital costs and ‘pain’ of implementing complex cleaning and sterilisation steps. Consequently, validation requirements are reduced whilst enabling rapid, scalable, and cost-effective phage manufacturing, helping innovators overcome critical production challenges.
Secondly, the adoption of standardised protocols for phage isolation, purification, characterisation, and formulation is critical. Harmonised methods, backed by advanced analytical tools, will ensure products consistently meet safety and efficacy requirements. Initiatives like Phagistry, an international clinical registry and data-sharing platform, are already laying foundations for more uniform approaches.¹

Thirdly, regulatory harmonisation is vital. Engagement between industry and agencies can help shape flexible frameworks that accommodate both standardised and bespoke phage therapies, fostering investment and accelerating approval pathways.6,7 Finally, dynamic public-private partnerships and collaborations will be key. Industry, academia, and healthcare systems must work together to expand phage banks, enhance screening platforms, and develop innovative business models that incentivise AMR-focused product development.
Real-world impact
Scaling phage therapy could have a significant real-world impact. In healthcare, it can reduce the incidence and severity of drug-resistant infections, lowering treatment costs, shortening hospital stays, and improving patient outcomes. For companies, early investment in phage manufacturing opens new market opportunities across therapeutics, diagnostics, and agriculture, diversifying portfolios and strengthening resilience in an evolving AMR landscape. Phage therapy also has the potential to support more use of antimicrobials, helping preserve the efficacy of existing antibiotics and promoting ecological balance in agriculture and aquaculture in the process.
On a global scale, the ability to manage resistant infections more effectively has the potential to save lives, improve food security, and strengthen public health systems. With AMR accelerating, it is vital that action is taken now. With strategic investment, regulatory innovation, and enabling technologies such as the CellMaker bioreactor platform from Cellexus, the life sciences community can help unlock the potential of phage therapy, hence being able to deliver sustainable, cost-effective solutions for patients, the industry, and society worldwide, making the idea of a new and better world much more than just a utopian idea but reality.
References
- World Health Organization (WHO). (2025). Building the evidence for the use of bacteriophage therapy. WHO Regional Office for Europe.
- Pirnay, J.P. et al. (2022). Phage therapy in Western Europe: Compassionate use and perspectives. Front. Microbiol.
- Dedrick RM et al. Engineered bacteriophages for treatment of a patient with a disseminated drug-resistant Mycobacterium abscessus. Nat Med. 2019 May;25(5):730-733. doi: 10.1038/s41591-019-0437-z. Epub 2019 May 8. PMID: 31068712; PMCID: PMC6557439.
- Celia Ferriol-González, Pilar Domingo-Calap (2021), Phage Therapy in Livestock and Companion Animals. [https://pmc.ncbi.nlm.nih.gov/articles/PMC8150778/]
- Clokie, M.R.J. et al. (2023). A bacteriophage cocktail delivered in feed significantly reduced Salmonella colonisation in broiler chickens. Emerg Microbes Infect, 12(1): 2217947.
- European Medicines Agency (EMA). (2023). Guideline on the quality, safety and efficacy of bacteriophage veterinary medicinal products.
- UK Medicines and Healthcare products Regulatory Agency (MHRA). (2025). Guidance for industry: Helping bring phage medicines to UK patients.
- Viertel, T.M., Ritter, K., & Horz, H.P. (2014). Viruses versus bacteria—novel approaches to phage therapy as a tool against multidrug-resistant pathogens. J Antimicrob Chemother, 69(9), 2326–2336.
- OECD (2023). Tackling antimicrobial resistance: Ensuring sustainable access to effective antimicrobials. OECD Health Policy Studies.
- The CDC website
Please note, this article will also appear in the 23rd edition of our quarterly publication.