AvantGuard’s AVA-003 compound offers a proactive solution against antimicrobial resistance, targeting pathogens with sustained activity, addressing the urgent need for innovative infection prevention in healthcare and conflict zones.
The global antimicrobial resistance (AMR) crisis, exacerbated by antibiotic overuse and slow drug development, has created an urgent need for alternative infection prevention strategies as multidrug-resistant pathogens proliferate in healthcare settings and conflict zones like Ukraine. AvantGuard’s new antiseptic compound, called AVA-003, offers proactive infection prevention through sustained antimicrobial activity. Unlike conventional antibiotics, AVA-003 demonstrates equivalent activity against antibiotic-susceptible and resistant bacteria/fungi via selective microbial cell wall targeting. This broad, non-specific mechanism prevents resistance development while enabling immediate decolonisation in a wound bed. AVA-003 represents a paradigm shift from reactive treatment to proactive prevention, providing a resistance-proof tool essential for managing the post-antibiotic era.
The challenges of modern wound treatment
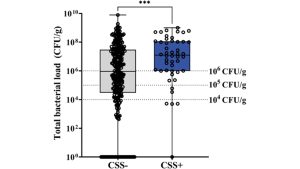
Imagine you are a doctor, and a patient presents with a mild cut after swimming in the ocean. You go through the standard clinical signs and symptoms checklist (CSS) and decide not to prescribe antibiotics. Unfortunately, the CSS consistently underestimates the amount of bacteria in wounds (Fig. 1),1 and your patient returns with a mildly infected wound, so you prescribe a first-line antibiotic, such as doxycycline, indicated for saltwater wound treatment. A week passes, and the treatment is not working; your patient’s wound is larger. You order cultures and resistance testing to treat it properly. This testing can take up to three days; by that time, the pathogen has spread in the patient’s community and within the hospital system to immunocompromised patients. You may find out that the pathogen is extremely susceptible to a different antibiotic, or it may not be. Maybe, worse still, there are two multidrug-resistant bacteria strains, with each strain susceptible to a different antibiotic; time is no longer on your side as the situation and patient worsen.
The global AMR crisis
We are losing the war against bacteria and fungi
Antimicrobial resistance is rapidly becoming one of the most pressing global health threats, driven by the overuse and misuse of standard antibiotics and compounded by poor hygiene and inadequate infection control. According to the World Health Organization, at least 1.27 million people die each year due to drug-resistant infections, and this number is projected to rise dramatically if current trends continue.2 This crisis is exacerbated by the alarmingly slow pace of new antibiotic development – currently, only a handful of innovative drugs are in progress for so-called priority pathogens. Priority pathogens are bacteria identified by health organisations as posing the greatest threat because they have developed resistance to multiple antibiotics. Likewise, multidrug-resistant pathogens are organisms that cannot be treated with several commonly used antibiotics, greatly limiting treatment options. As resistance rates climb and effective therapies dwindle, healthcare systems worldwide face the harsh reality of being unable to contain or treat the spread of these dangerous infections.
Healthcare providers now face impossible choices. When first-line antibiotics fail against these multidrug-resistant pathogens – organisms that shrug off several commonly used drugs – clinicians must escalate to last-resort treatments that often have complicating side effects. Each use of these powerful weapons breeds more resistance, accelerating the very crisis they’re meant to address. The widespread reliance on broad-spectrum antibiotics underscores the urgent need for alternative strategies to control resistant pathogens.
Ukraine has become ground zero for the post-antibiotic future
Combat-related heavy metal poisoning is a potential contributing factor to further intensifying antimicrobial resistance among bacterial populations.3 Added to this are the profound challenges in maintaining hygiene for soldiers and the increase in untreated injuries amid ongoing conflict, which together foster the unchecked expansion of antibiotic-resistant pathogens.
With limited resources to identify resistance patterns, maintain sanitation protocols, and access appropriate antibiotics, healthcare providers in these environments face formidable obstacles. Pathogens are not only spreading within hospitals but also creating biofilms on medical equipment and wounds, leading to systemic infections that are challenging to eradicate.
Concrete examples highlight the gravity of the situation. For instance, pan-resistant strains of Klebsiella pneumoniae have been isolated from wounds in Ukrainian soldiers. These hypervirulent strains were documented in US military hospitals in Europe that treat Ukrainian patients, underscoring the global reach of the problem.4
The post-antibiotic world is already here. These developments signal the urgent need for effective, alternative infection prevention strategies, as conventional antibiotics become increasingly unreliable in the face of rapidly evolving resistance.
Prevention is the best cure
Antiseptics provide an alternative strategy to antibiotics, attacking pathogens through broad mechanisms that do not easily allow bacteria to develop resistance. Rather than targeting a single bacterial process, they disrupt multiple vital systems at once, making it far more difficult for organisms to adapt and survive.
However, the practical limitations of current antiseptics cannot be overlooked. For instance, agents like chlorhexidine and iodine are widely used in clinical and surgical settings to disinfect skin, wounds, and medical equipment due to their strong antimicrobial properties. Yet, their effectiveness comes at a cost: they can cause skin irritation or delay healing when overused or applied to sensitive tissues.5,6 This trade-off – potent pathogen control balanced against potential harm to human tissue – has restricted their use to specific scenarios, leaving a significant gap in our infection prevention arsenal at a time when we need broad, safe solutions more than ever.
AVA-003: Breaking the cycle of resistance
AvantGuard has engineered a paradigm shift in infection control
AVA-003 represents the first antiseptic to solve the fundamental trade-off that has plagued infection prevention for decades: the choice between effectiveness and safety. This breakthrough formulation – available as both liquid and hydrogel – delivers unprecedented and sustained antimicrobial activity compared to all other antiseptics and topically-applied antibiotics.
The data tells a compelling story
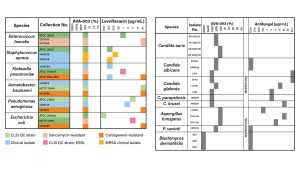
While traditional antibiotics and antifungals show wildly inconsistent results against different pathogens (Figs. 2 and 3), AVA-003 demonstrates uniform lethality. Resistant or susceptible, bacteria or fungi – it makes no difference. AVA-003 kills them all with equal efficiency, eliminating the guesswork that has made empirical therapy a gamble with patient lives.
Safety without compromise
AVA-003’s selective targeting mechanism preferentially attacks microbial cell walls rather than human tissue. This precision enables a broad range of applications for the same compound, i.e., disinfecting surfaces, sterilising surgical instruments, and treating wounds without fear of tissue damage or healing delays, that would otherwise be impossible with weaker or toxic alternatives. The result is a single agent that can sanitise entire healthcare environments while being gentle enough for direct patient application.
Consider the clinical transformation
That same patient presents with a contaminated ocean wound, but now, instead of the familiar cascade – underestimate bacterial load, prescribe antibiotics, watch resistance emerge, escalate treatment, spread resistant pathogens – you simply wash the wounds with an AVA-003 containing liquid and apply an AVA-003 gel. The wound heals cleanly. No resistance develops. No follow-up visits required. The community stays safe, and healthcare resources are preserved for true emergencies.
In combat zones, AVA-003 becomes a force multiplier
Field medics can neutralise dangerous pathogens at the point of injury, preventing the transport of resistant organisms back to military hospitals. What once seeded epidemics of pan-resistant bacteria becomes contained at the source, protecting both individual soldiers and entire healthcare systems.
This is more than incremental improvement – it’s infection prevention reimagined

As we race toward a post-antibiotic future where pan-resistant bacteria and fungal pandemics threaten global health security, AVA-003 offers the only sustainable path forward: stopping infections before they start, with zero risk of resistance development. The future of infection control isn’t about finding new ways to kill superbugs – it’s about preventing them from emerging at all.
References
- Serena, T. E.; Gould, L.; Ousey, K.; Kirsner, R. S. Reliance on Clinical Signs and Symptoms Assessment Leads to Misuse of Antimicrobials: Post hoc Analysis of 350 Chronic Wounds. Advances in Wound Care 2021, 11 (12), 639–649. DOI: 10.1089/wound.2021.0146 (acccessed 2025/09/10).
- Murray, C. J. L.; Ikuta, K. S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. The Lancet 2022, 399 (10325), 629–655. DOI: 10.1016/S0140-6736(21)02724-0 (accessed 2025/09/10).
- Balta, I.; Lemon, J.; Gadaj, A.; Cretescu, I.; Stef, D.; Pet, I.; Stef, L.; McCleery, D.; Douglas, A.; Corcionivoschi, N. The interplay between antimicrobial resistance, heavy metal pollution, and the role of microplastics. Front Microbiol 2025, 16, 1550587. DOI: 10.3389/fmicb.2025.1550587 From NLM.
- Ljungquist, O.; Magda, M.; Giske, C. G.; Tellapragada, C.; Nazarchuk, O.; Dmytriiev, D.; Thofte, O.; Öhnström, V.; Matuschek, E.; Blom, A. M.; et al. Pandrug-resistant Klebsiella pneumoniae isolated from Ukrainian war victims are hypervirulent. J Infect 2024, 89 (6), 106312. DOI: 10.1016/j.jinf.2024.106312 From NLM.
- Wang, D.; Huang, X.; Lv, W.; Zhou, J. The Toxicity and Antibacterial Effects of Povidone-Iodine Irrigation in Fracture Surgery. Orthop Surg 2022, 14 (9), 2286–2297. DOI: 10.1111/os.13422 From NLM.
- Fiorillo, L.; D’Amico, C.; Mehta, V.; Cicciù, M.; Cervino, G. Chlorhexidine cytotoxicity on oral Behaviors: Last 20 Years systematic review. Oral Oncology Reports 2024, 9, 100245. DOI: https://doi.org/10.1016/j.oor.2024.100245.
Please note, this article will also appear in the 24th edition of our quarterly publication.