Following her successful application for an ERC Starting Grant, Simona Huwiler discusses the potential of Bdellovibrio bacteriovorus, a predatory bacterium, to address the growing concern of antibiotic resistance.
The public health implications of antimicrobial resistance are concerning. A recent study by the World Health Organization (WHO) in its 2025 Global AMR Surveillance Report indicated that 1 in 6 bacterial infections worldwide in 2023 were resistant to antibiotics. Another study examining data from 2019 reported that approximately 5 million deaths were associated with AMR. Among these, around 3 million deaths were directly attributable to antimicrobial resistance.
This widespread occurrence of antimicrobial resistance makes it increasingly difficult to treat bacterial infections in hospitals, while also posing a threat to various medical procedures such as surgeries and cancer treatments.
Currently, the goal is to prevent the inappropriate use of antibiotics, particularly in cases where they are ineffective. Many governments are working to restrict the use of last-resort antibiotics in animal farming, as they are essential for treating certain infections. In some countries, antibiotics can be obtained without a prescription and can often be misused for viral infections, while efforts are made to prevent this.
A potential solution: Bdellovibrio bacteriovorus
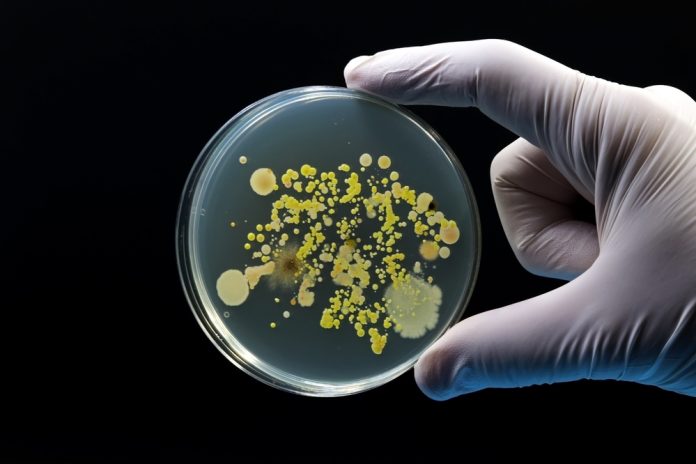
Bdellovibrio bacteriovorusis a ‘living antibiotic’ – a predatory bacterium that has intrinsic energy, allowing it to be agile and capable of actively seeking other bacteria. It attaches itself to other gram-negative bacteria on the outside, then creates a hole in the outer membrane and the peptidoglycan cell wall, allowing it to enter its prey bacterium. Inside, it consumes the prey, divides, and then, the new predator cells exit the prey bacterial remnants. In contrast, traditional antibiotics generally work by diffusion until they reach their target bacteria, which they then kill.
This predatory bacterium primarily kills and feeds on other gram-negative bacteria, many of which are included in the WHO Bacterial Priority Pathogens List, such as carbapenem-resistant Klebsiella pneumoniae and Escherichia coli. Understanding the mechanisms this predator uses to damage and kill its prey could lead to the development of new drugs to tackle AMR.
One disadvantage of these ‘living antibiotics’ is their larger size compared to the small molecules that make up conventional antibiotics. This limitation may restrict their future applications primarily to treating surface infections, such as those found in wounds or the mucosal surfaces of the lung and gut. Because these ‘living antibiotics’ are too large to penetrate deeply into tissues, they may not be effective against infections located deeper within the tissue, like intracellular infections, where traditional antibiotics are superior.
However, B. bacteriovorus has multiple mechanisms involved in the attachment and elimination of its bacterial target. Traditional antibiotics typically have a single mechanism of action to kill bacteria, making it easier for pathogens to evolve resistance. In contrast, the varied mechanisms of ‘living antibiotics’ could make it more challenging for bacteria to adapt and develop resistance.
Promising safety data
Many available studies indicate that B. bacteriovorus is safe and effective to clear many infections caused by gram-negative pathogenic bacteria. A substantial amount of research has been conducted in the past and is ongoing, demonstrating its efficacy even against bacteria that are resistant to antibiotics. This effectiveness appears to be independent of whether the bacteria have antibiotic-resistant genes.
The studies conducted include various standard animal models, such as mice, rats, and zebrafish. However, there may be a need for more advanced models, potentially involving differentiated human cell cultures and organoids, to better simulate human conditions. Studies involving human cells have been conducted to evaluate toxicity, and findings suggest that it is well-tolerated.
Timeline to widespread applications
Currently, clinics in the Western world generally have fast diagnostics in place to identify alternative antibiotics for cases of multiple antibiotic resistance. Therefore, there is no immediate, urgent need in the clinics in most cases. However, the urgency is likely to increase in the future as there is a steady increase in antibiotic-resistant infections and the population in the Western world gets older and more susceptible to infections.
At present, a non-medical product containing B. bacteriovorus is being sold in the UK and is expanding into the EU market, to protect and re-establish the intimate microbiome. Although it has already undergone some testing, blind and controlled studies are still needed, which is an important step towards medical approval.
A key future goal is to manufacture a product with B. bacteriovorus under Good Manufacturing Practice (GMP) standards. This will ensure that these predatory bacteria can be consistently produced in a safe and controlled manner, making it suitable for human medical use. With GMP-compliant products, clinical trials to evaluate effectiveness can be initiated, comparing the outcomes of a real treatment and a placebo group.
Looking ahead, one of the first applications of this treatment may be in situations where conventional methods fail. This type of experimental treatment or compassionate use could be administered to patients dealing with multidrug-resistant bacterial strains, provided they agree and the necessary framework is in place.
Another avenue for advancing this potential new therapy based on B. bacteriovorus is its use in chronic infections, such as cystic fibrosis, where patients require long-term or frequent antibiotic treatments. This approach may help alleviate some unpleasant side effects associated with some antibiotics. In cases where a patient is infected with multidrug-resistant pathogenic bacteria, treatment with bacteriophages (bacterial viruses), or potentially with B. bacteriovorus, could be a last-resort option.
Additionally, there are efforts to produce Bdellovibrio in an encapsulated form, particularly for use in agriculture and aquaculture. Bdellovibrio has been shown to combat bacterial pathogens affecting shrimp and is used in shrimp aquaculture. In agriculture, it is being explored as a means to prevent diseases affecting crops, such as potatoes. However, it is important to note that this method is only effective against certain bacterial plant pathogens, and many plant diseases are caused by fungi. Nevertheless, in human medicine, bacterial plant pathogens pose a significant concern.
Remaining questions and barriers
The primary research focus going forward should be on differentiated human cells and organoid models, which bring us closer to real-world applications in humans. There is hope that, in the future, we might be able to engineer these predatory bacteria to target only pathogenic bacteria while leaving beneficial bacteria unharmed.
A significant question still largely remains regarding how these predatory bacteria interact with the microbiome from an ecological perspective. In very low numbers, predatory bacteria are found in most microbiomes; however, we have yet to understand how introducing more of them could impact the microbiome. With most antibiotics, we often kill a significant portion of the microbiome without giving it much thought. In contrast, phage therapy utilises viruses that target specific bacteria, which typically preserves the microbiome. However, it requires precise diagnostics to select an appropriate phage.
Further, we must consider the challenges posed by the regulatory landscape. For antibiotics, there are standard requirements and procedures to reach approval from agencies like the European Medicines Agency (EMA). This process could be challenging when applying the same framework to B. bacteriovorus, as it is a living organism. Fortunately, there is an opportunity for dialogue with EMA, for example, regarding phage therapy. If there is an unmet medical need, there may be ways to navigate some standard regulatory procedures. In particular, studying the regulatory framework for probiotics could be beneficial, as probiotics are also bacteria, and recently, some probiotic products received approval by the U.S. Food and Drug Administration (FDA). In general, it is expected that the timeline for obtaining approval for these ´living antibiotics´ will likely be much longer than that for standard antibiotics, based on their unique properties.
Securing funding for fundamental research on predatory bacteria through an ERC Starting Grant project
As of the 10th of November 2025, the Swiss-EU Programmes Agreement (EUPA) has been signed in Bern, retroactively associating Switzerland with Horizon Europe. This enables my ERC Starting Grant 2025 project investigating the interaction of predatory bacteria with prey bacteria to be executed at a Swiss University, namely the University of Bern. Unfortunately, this was not possible for the ERC Starting Grant 2024 call. The EUPA are part of the Bilateral Agreements III between the EU and Switzerland, and based on my perspective, very important for Swiss science and innovation.
Please note, this article will also appear in the 24th edition of our quarterly publication.