Erik Nijkamp and Paul Volčokas from Team Better/e, a student team from Eindhoven University of Technology (TU/e), aim to pave the way for eco-friendly, cost-effective redox flow batteries, able to power entire cities in times of insufficient supply of renewable energy.
The fact that the current generation of TU/e students is driven more than ever by societal challenges is well-illustrated by the many student teams that the university houses. Even major global events like the COVID-19 pandemic cannot stop these young, smart, and passionate people from chasing their dreams to make a difference.
Here, Erik Nijkamp and Paul Volčokas from Team Better/e, a student team that started in the midst of the pandemic, share their plans and ambitions for the future.
Erik Nijkamp, who is currently studying for his Masters in Chemical Engineering and founded Team Better/e with two other students, said: “In 2020, during the pandemic, I joined the TU/e Honors Academy.
“I immediately knew that I didn’t want to join an existing student team, but that I wanted to develop my own project. After extensive talks with staff members, I decided to look into the potential of liquid metal batteries.”
Paul Volčokas added: “Whilst in my second year as a mechanical engineering student, I was presented with the idea of the liquid metal battery. Since the combination of fluid flows and heat management intrigued me, I decided to join the team.”
A liquid metal battery takes normally solid metals, such as tin and calcium, and heats them up to 600°C to turn them into liquids. The metal then releases electrons which can be collected to produce energy. During charging, the electrons are reunited with the metal ions, resulting in a neutral metal again. These processes lead to a highly efficient, rechargeable battery.
Making a switch: Developing redox flow batteries
After an extensive research phase that lasted about a year, the team decided to discard its initial idea of working on liquid metal batteries.
Nijkamp said: “On paper, that idea looks great. It should be super cheap and efficient. And since the design doesn’t rely on membranes, in theory, such a battery could last forever.”
But, as is so often the case, practice turned out to be more complicated.
“Liquid metals are hot. And to prevent the metal from rusting, the environment has to be completely free of oxygen. Such a device is way too hard for students to fabricate,” Nijkamp continued.
Volčokas said: “We understood that, at the university, we both lack the required skills and the permits to conduct the necessary experiments on liquid metals. So, we decided to switch to redox flow batteries. This is a technology that already exists in the lab. And in China, a specific type of redox flow battery is produced at a commercial scale already, though not yet for grid-sized applications.”
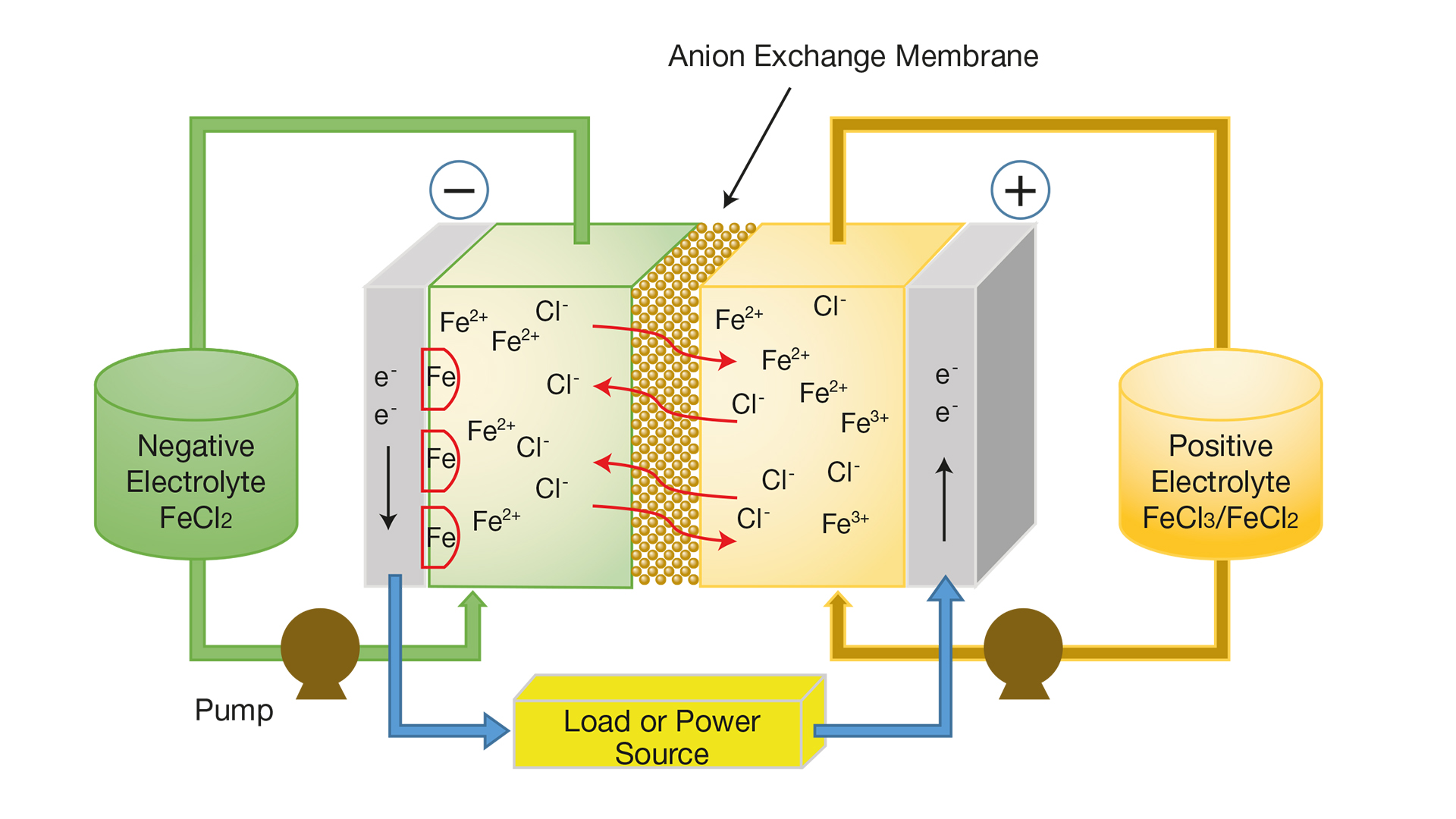
This first generation of commercially available redox flow batteries is based on vanadium, which is a rare earth metal. Team Better/e explicitly aims to develop a more eco-friendly and cost-effective alternative based on iron, which is abundantly available.
On paper, the working mechanism of their redox flow battery is fairly straightforward.
Redox flow batteries consist of two tanks, a positive tank and a negative tank, separated by a core where chemical reactions take place. The positive tank contains FeCl2, and the negative tank contains FeCl3. Two reactions occur in the core.
While discharging, Fe is converted to Fe(2+), which means electrons will be released. On the other side, Fe(3+) is converted to Fe(2+), which will use electrons. While charging, both reactions go in the opposite direction.
The size of the tanks is directly proportional to how long you will be able to power your home or factory. And the size of the core is directly proportional to the number of buildings the battery can support.
Until recently, due to problems with the required membranes, redox flow batteries only were considered a realistic probability for small-scale applications.But in recent years, membrane technology has significantly improved, for example with the research of TU/e professor Antoni Forner-Cuenca.
Nijkamp said: “We are convinced that redox flow battery technology can be made ready for large-scale applications as well. We want to accelerate this development, by making everything we do available open source.”
Two teams to tackle problems
Nijkamp said: “We started by trying to reproduce a design from a paper. But that didn’t work at all.”
Volčokas continued: “We got confronted with massive leakages, since the original design used clamps instead of bolts. So we have been working on a new design for the housing, which we are currently testing for leakages.”
Within Team Better/e, two teams have been established. One team is focused on the physics of the cell housing, and the second team is working on the battery chemistry.
Nijkamp said: “One of the chemical challenges we will have to tackle is how to fight the hydrogen evolution reaction that occurs when the pH is too low and hydrogen gas is formed. Another problem we have to solve is that sometimes solid iron is formed, which forms dust and leads to slurry in our tanks.”
“What is worse is that solid iron can form dendrites, which puncture our membrane and cause safety issues,” Volčokas added.
The first aim of the team is to demonstrate that their idea works on a single cell level.
Volčokas explained: “When we’ve achieved that, we will build a small-scale demonstrator for Eindhoven Airport, with whom we have a partnership, to show the public what we are working on. And then the next step is to develop our single cell into a stack of cells.”
Nijkamp said: “I like to dream big. It would be great if eventually Team Better/e could build a battery that can power a building on the university campus.”

Innovating redox flow batteries for the future
All in all, the students still have a lot of technological challenges to overcome. But they are optimistic.
“We get a lot of help here at the university, especially from Antoni Forner-Cuenca and his group. The Honors Academy has also been of great help, as well as our coaches, Yali Tang and Mark Cox. During the process, EIRES has been one of our biggest supporters. Whether it’s providing us with a meeting space or sending us to Denmark to compete in an international event, EIRES has always been helping us. And we work closely with the FAIR battery project, which is led by Dr Sanli Faez from Utrecht University, and where Antoni Forner-Cuenca and Yali Tang from TU/e are involved. Additional to providing us with financial support, they shared all of their information with us, up until the electronic specifications and the blueprint of their design,” Nijkamp continued.
It is this open innovation attitude both students are also advocating themselves.
“We do not want to make a profit out of this, but we want to lower the barrier for entry by advancing this technology to such a level that others are interested to step in and take over,” added Nijkamp.
To achieve this ambition, new members are more than welcome to join the team, both students emphasise.
Volčokas said: “By the end of this academic year, some of the current team members will leave the team. So we can certainly use some new people. There are lots of challenges that still need to be tackled, for example when it comes to powering the stack.”
“And if any academic staff members can help us in gaining access to the high-quality materials we need, that would be most welcome too,” Nijkamp added.
For both students, so far the experience of being part of Team Better/e has been a very joyful and fruitful one. Volčokas said: “The goal of the Honors Academy is to grow beyond the curriculum and gain new skills. Over the past year, we have entered in multiple contests and competitions, where we had to present our ideas to huge crowds and some important people. That requires skills I would have never gained otherwise.”
Nijkamp concluded: “My goal in life is to leave things better than I found them. It would be great if we could end up with a result that other people can build on to make large-scale battery storage a reality.”
For more information, visit:https://www.teambettere.com/
Please note, this article will also appear in the fifteenth edition of our quarterly publication.