Researchers at the California NanoSystems Institute have made a discovery that could lead to better, safer lithium-metal batteries that outperform current lithium-ion batteries.
Lithium-ion batteries are more commonly used in modern technologies; however, they descend from lithium-metal batteries, which have the potential to hold double the energy.
Despite their great potential, these batteries haven’t been developed as broadly due to the risk of them catching fire or exploding.
The new research, detailed in a paper in Nature, has the potential to commercialise these batteries and make them safer for use.
Discovering the true shape of lithium metal
Metallic lithium reacts so quickly with chemicals that, under normal conditions, corrosion forms almost immediately while the metal is laid down on a surface such as an electrode.
This prevents the common use of lithium-metal batteries in everyday technologies such as phones and watches.
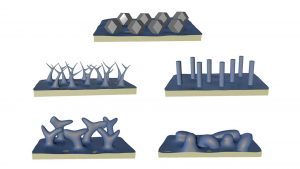
The researchers developed a technique that prevented corrosion and showed that, in its absence, lithium atoms assemble into a surprising shape — the rhombic dodecahedron, a 12-sided figure.
Yuzhang Li, assistant professor of chemical and biomolecular engineering at UCLA and the study’s corresponding author, explained: “There are thousands of papers on lithium metal, and most structure descriptions are qualitative, such as ‘chunky’ or ‘column-like’.
“It was surprising to discover that when we prevented surface corrosion, instead of these ill-defined shapes, we saw a singular polyhedron that matches theoretical predictions based on the metal’s crystal structure.
“Ultimately, this study allows us to revise how we understand lithium-metal batteries.”
Can the metal be shaped to produce safe lithium-metal batteries?
At tiny scales, lithium-ion batteries store positively charged lithium atoms in a cage-like structure of carbon that coats an electrode.
In contrast, lithium-metal batteries coat the electrode with metallic lithium. This packs ten times more lithium into the same space than lithium-ion batteries, accounting for the increase in both performance and danger.
The process for laying down the lithium coating is based on a 200-plus-year-old technique that employs electricity and solutions of salts called electrolytes. Often, the lithium forms microscopic branching filaments with protruding spikes.
If two of those spikes crisscross in the battery, it can cause a short circuit that could lead to an explosion.
The revelation of the true shape of lithium suggests that the explosion risk for lithium-metal batteries can be abated because the atoms accumulate in an orderly form instead of one that can crisscross.
“Scientists and engineers have produced over two decades’ worth of research into synthesising metals including gold, platinum, and silver into shapes such as nanocubes, nanospheres, and nanorods,” Li said.
“Now that we know the shape of lithium, we need to know whether we can tune it so that it forms cubes.”
A new technique for depositing lithium
Until now, the prevailing view had been that the choice of electrolytes in solution determines the shape that lithium forms on a surface – whether the structure resembles chunks or columns. The UCLA researchers had a different idea.
“We wanted to see if we could deposit lithium metal so quickly that we outpace the reaction that causes the corrosion film,” said UCLA doctoral student Xintong Yuan, the study’s first author.
The researchers developed a new technique for depositing lithium faster than corrosion forms. They ran current through a much smaller electrode to push electricity out more quickly.
A balance was required, however, because speeding up the process too much would lead to the same spiky structures that cause short circuits.
The team addressed that issue by adjusting the shape of their tiny electrode.
They laid lithium on surfaces using four electrolytes, comparing results between a standard technique and their new method. With corrosion, the lithium formed four distinct microscopic shapes deemed safe for the commercial use of lithium-metal batteries.
However, their corrosion-free process found that the lithium formed minuscule dodecahedrons no bigger than two-millionths of a metre in all four cases.









