How French researcher Jérôme Galon and Immuno-Oncology diagnostics company HalioDx are tackling the need for patients’ stratification for cancer treatment.
Despite considerable social awareness and research effort, cancer remains one of the leading causes of morbidity and mortality worldwide. Nonetheless, impressive advances have been made since the early 2000s, fundamentally revealing the power of patients’ own immune systems to fight and possibly defeat the disease. Dr Jérôme Galon, an INSERM First-Class Research Director at the Centre de Recherche des Cordeliers in Paris, France, was one of the pioneers in the field of what is currently known as Immuno-Oncology. In 2006, he first demonstrated the importance of the type, density, and location of immune cells within human tumors in predicting patients’ survival and clinical outcome, regardless of the local extent and spread of the tumour, strongly supporting the nowadays confirmed hypothesis that the immune system shapes human tumors.1 Dr Galon also defined the so-called ‘immune contexture’, a series of immune-related parameters determining cancer evolution. This previously undescribed strength of the immune reaction changed the understanding of cancer evolution and has important consequences in clinical practice. In recognition of this ground-breaking research, Dr Galon was awarded with several international prizes, including the William B. Coley Award in 2010, which honors the best scientists in fundamental and cancer immunology.
The era of cancer immunotherapies: progress and challenges
A further milestone for cancer patients has been reached in 2013, with the acknowledgement of the value of cancer immunotherapy.2 The excitement is mainly based on the observation that this approach is unleashing the pre-existing immunity of the patients and can result in complete remission in patients with advanced solid cancer, which is unique. Since then, immunomodulatory antibodies, such as the anti-PD (L)1, are becoming the reference treatment in a growing number of indications. However, the limited percentage of responding patients (approximately 25%) highlighted the need for more personalised approaches, as well as that for deeper fundamental knowledge. A hunt for the best predictive biomarkers (i.e. any substance, structure, or process that can predict the response to a specific therapy) is ongoing: identifying responding patients in advance would reduce unnecessary, often non-negligible therapy-associated risks for the patients, as well as the financial burden for the whole society. In fact, the estimated cost of immunotherapy (several hundred thousand euros per patient) can only be sustainable in the medium term with an effective patient selection.
Towards the introduction of Immunoscore® in cancer classification
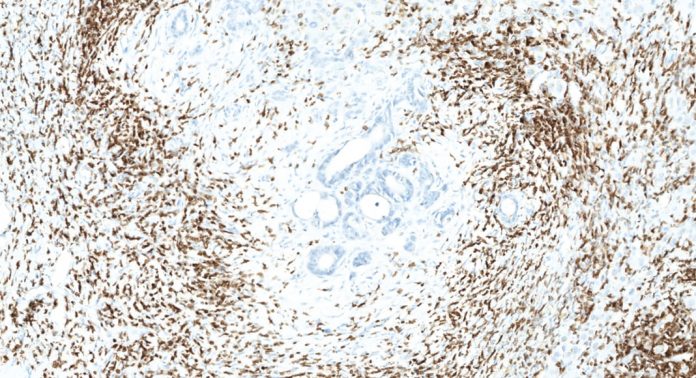
The discovery of the central role of the immune system in the battle against cancer must be accompanied by a revision of the classical tumor-centric paradigm. Perhaps one of the most urgent revisions involves an update of the current cancer classification. The American Joint Committee on Cancer/Union Internationale Contre le Cancer (AJCC/UICC)-TNM classification is the most common system for classifying the degree of tumour progression and invasion and has been used for over 80 years.1 Albeit powerful, the TNM classification proved unable to provide complete prognostic information, as clinical outcome can significantly vary among patients within the same tumour stage. A major limitation of this system is that it relies mostly on tumor intrinsic features and does not consider the effect of the host immune response on tumor development. Amongst all the proposed alternative methods, only one demonstrated higher prognostic power over the TNM classification and is based on the analysis of the tumour-associated immune response: the Immunoscore.1,4 The Immunoscore was conceived and developed by Dr Galon and his team in the light of his ground-breaking findings. Immunoscore consists in an Immunohistochemistry (IHC) – and digital pathology-based scoring system reflecting the presence of certain subtypes of immune cells, namely CD3 and CD8 lymphocytes, within specific regions of the tumour (its centre and its border, termed invasive margin).5 The scoring ranges from low (Immunoscore 0, I0) to high immune cell densities in both locations (Immunoscore 4, I4). Compared to the traditional TNM system, the Immunoscore is the only test better predicting survival for patients with non-metastatic (Stage I-II-III) colorectal cancers.1,4
In 2012, Dr Galon initiated a Worldwide Immunoscore Consortium to promote the utilisation of the Immunoscore in routine clinical settings, with the support of the Society for Immunotherapy of Cancer (SITC),6,7 the results of which have just been published in The Lancet in May, 2018.8 By involving international expert pathologists and immunologists from twenty-three international centres, the Consortium successfully identified a strategy to demonstrate the feasibility and reproducibility of a consensus Immunoscore, validated its major prognostic power in colon cancer stage I/II/III, and demonstrated the utility of consensus Immunoscore to predict stage II colon cancer patients with high-risk of recurrence. It is hoped that this initiative will result in the implementation of consensus Immunoscore as a new component for the classification of cancer TNM-I (I for Immune).
This may have profound practical implications and impact. For example, in routine clinical practice, the treatment for patients with localised colorectal cancers (not involving lymph nodes or distant sites) consists in surgical removal of the tumour. However, the presence of undetected metastases cannot be excluded, even at this early stage. In fact, this might be the case for these patients (approximately 25%) having disease recurrence. This subgroup of patients could benefit from additional therapies, which could extend their life expectancy. Strikingly, whilst the current classification fails to identify this subgroup, the Immunoscore approach can. Compelling evidence demonstrated that the majority of patients with a low score experienced tumour recurrence and had a significantly lower life expectancy than patients with high score. Masterfully, by the means of the Immunoscore, digital pathology and artificial intelligence are first stepping into the emerging field of immuno-pathology.
HalioDx: pioneering Immuno-oncology diagnostics
To translate the potential of the Immunoscore to the clinical setting, in 2015 Dr Galon co-founded a biotech company specialised in immuno-oncology diagnostics, HalioDx, for which he still serves nowadays as consultant and as chairman of the Scientific Advisory Board. HalioDx headquarters are located in Marseille, France and a US subsidiary, HalioDx Inc. has just opened. Indeed, HalioDx achieved validation of standardised procedures for Immunoscore, and successfully developed a robust, standardised consensus method compliant with in vitro diagnostic requirements. The resulting assay, termed Immunoscore Colon, is the first immune in vitro diagnostic assay to assess the risk of relapse in colon cancer patients. Under its CE-IVD version, it represents the first immune scoring diagnostic tests used in routine by pathology labs leveraging advanced image analysis. HalioDx received two awards in 2016 for its accomplishment, the ‘Seal of Excellence Certificate’ by the European Commission and the award of the ‘Worldwide Innovation Challenge.’ Dr Galon and HalioDx are currently aiming at validating the predictive and prognostic value of the Immunoscore in other cancer types.
HalioDx is rapidly placing itself at the forefront of Immuno-oncology diagnostics with a range of tools and a rich pipeline of proprietary
immuno-oncology diagnostic applications, including but not limited to the Immunoscore. Another CE-IVD approved, standardised Immunohistochemistry (IHC)-based assay, Halioseek® PD-L1/CD8, was developed for the detection of PD-L1 protein and the concomitant detection of CD8 + cells in non-small cell lung cancer (NSCLC) tissue. This test aims to help identifying which NSCLC patients may best respond to anti-PD1 or anti-PD-L1 immunotherapies. HalioDx is committed to further leverage the immune contexture concept and to identify robust predictive biomarkers to guide therapeutic regimes. By providing immuno-oncology diagnostics tools, companies like HalioDx have the potential of transforming cancer patients’ management. It seems increasingly clear that the complexity of the immune response to tumours calls for intensifying and combining academic and industry efforts to better understand the biology of the immune response to cancers and provide validated assays for patients.
References
- Galon, J. et al. Science 313, 1960-4 (2006).
- McNutt, M. Science 342, 1417 (2013).
- Locker, G.Y. et al. J Clin Oncol 24, 5313-27 (2006).
- Mlecnik, B. et al. J Clin Oncol 29, 610-8 (2011).
- Pages, F. et al. J Clin Oncol 27, 5944-51 (2009).
- Galon, J. et al. J Pathol 232, 199-209 (2014).
- Galon, J. et al. J Transl Med 10, 1 (2012).
- Pages, F. et al. Lancet 391, 2128-2139 (2018).
Jérôme Galon
Director of Research
Chief Inserm laboratory
Laboratory of Integrative
Cancer Immunology
INSERM UMRS1138, Cordeliers Research Centre
+33 1 44 27 9085
jerome.galon@crc.jussieu.fr