Non-muscle-invasive bladder cancer has a 60-70% chance of reoccurring in patients – it’s time to talk about it.
Understanding and overcoming cancer progression and therapy resistance is a major overarching goal of cancer research. It is true that much progress has been made in various cancer fields (surgery, chemotherapy, radiotherapy, together with immunotherapy and targeted therapy), but still the absolute number of annual cancer diagnoses and related deaths is projected to increase by 2030.1 At the Urology Research Laboratory of the Department for BioMedical Research, our main research focus is genitourinary cancer, mostly prostate and bladder cancer.
Prostate cancer
Prostate cancer is the most frequently diagnosed malignancy and second leading cause of cancer-specific deaths in men in Western countries.2 Clinically, prostate cancer is highly heterogeneous; manifestations vary from indolent localised tumours to widespread metastases. Prostate cancer is one of the most prevalent cancer to metastasize to the bone – approximately 70% of advanced prostate cancer patients are diagnosed with bone metastases.3 Together with breast cancer, prostate cancer accounts for more than 80% of cases of bone metastatic disease. The presence of bone metastasis is associated with a number of skeletal-related events, such as fractures, nerve compression and pain which can complicate the patient’s management.4 It is well accepted that the progression to castration-resistant prostate cancer (CRPC) is directly linked to metastatic prostate cancer, which is a lethal disease.
Radical prostatectomy is mostly performed in patients with a localised disease (and in a few oligometastatic patients) so that metastatic disease and their tumours are generally not available for molecular analysis. Molecular characterisation of prostate cancer has revealed striking genomic heterogeneity and defined distinct molecular subclasses that may provide insight to the variable clinical course. The frequency of the most common alteration in prostate cancer is low (between 4% to 10% for TP53, PTEN, SPOP, KRAS and EGFR) or even lower for other tumour drivers FOXA1 (3%), BRAF (2%) and PI3kCA (2%).5-8
Moreover, a promising maker associated with early onset of prostate cancer is TMPRSS2-ERG.9,10 However, ERG immunohistochemistry is not predictive for prostate specific antigen (PSA) recurrence or overall survival after radical prostatectomy or following radiotherapy.11-13 Despite the fact that genomic studies have characterised these genetic alterations, their prognostic significance remains unclear and as a result, complicates risk stratification and management strategies for prostate cancer patients. Therefore, there is an urgent need to find new prognostic biomarkers to improve prostate cancer patient stratification and achieve personalised treatments.
Bladder cancer
Urothelial carcinoma of the bladder (BLCa) is the fifth most common cancer in the Western world.14,15 A small proportion of patients are diagnosed with muscle-invasive bladder cancer (MIBC), whereas the majority (75%) is diagnosed with non-muscle-invasive bladder cancer (NMIBC). NMIBC is treated by transurethral resection of the bladder tumour (TURBT). Patients with low grade NMIBC receive one cycle of early instillation chemotherapy into the bladder. Those with high grade NMIBC undergo a second TURBT to confirm the complete resection of the tumour.
Within six to seven weeks after diagnosis, these patients receive BCG (Bacillus Calmette-Guérin) instillations into the bladder to elicit an immune response as an adjuvant treatment.16 NMIBC rarely progresses to invasive or metastatic BLCa. However, overall NMIBC has a high rate of recurrence (60-70%) within one year of diagnosis.16 Even patients with high grade NMIBC treated with BCG have a high risk of cancer recurrence (30-40%).17,18 Consequently, patients with low grade NMIBC will be followed by cystoscopies every six months for five years. Whereas patients with high grade NMIBC are followed with lifelong with cystoscopy. These expensive and invasive follow-ups make NMIBC the costliest cancer to treat on a per patient basis.15 Despite the prevalence, the high costs, the high patient discomfort and the severe socio-economic burden of NMIBC, this cancer is still relatively understudied.19
The mutational burden of NMIBC is low, TP53 and RB1 mutations as well as APOBEC mutagenesis are less frequent than in MIBC, while activating FGFR3 mutations are found in 57% of NMIBC.20-22 NMIBC is highly heterogeneous both genomically and transcriptomically.20,21 Interestingly, researchers have investigated tumour specimens of metachronous BLCa recurrences in the same patient during a follow-up. They showed evidence for high clonal similarities between the initial and recurrent NMIBC.23 These findings may be explained by the clonality of multifocal NMIBC as well as field cancerisation in the bladder determined by whole organ mapping of NMIBC patients.24-26 Field cancerisation even within the morphological normal bladder is thought to be the main reason for the high recurrence rate of NMIBC.27 However, the molecular landscape of NMIBC and clonality of cancer recurrence has not yet been used for a personalised adjuvant instillation into the bladder of high grade NMIBC or its clonal recurrence. The remarkable level of intra and inter-patient heterogeneity observed in both prostate and bladder cancer strongly supports the need for a more personalised approach from which both patient stratification and treatment can benefit.
Patient derived organoids
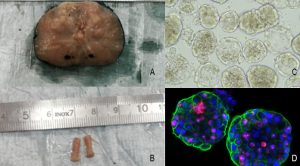
In cancer research, one of the greatest hurdles is the lack of omni-comprehensive models to untangle drug response prediction and resistance on a patient to patient basis. Animal models have been widely used to test highly focused questions, however their use in high throughput experimental settings is clearly limited. 2D cell lines, although very practical and easy to handle, lack the heterogeneity observed in vivo. A relatively new promising model are patient derived organoids (PDO), which are small 3D multicellular structures that can be culture in vitro and also used to study individual mechanisms of tumour formation and drug response. Differently from 2D cell lines, PDOs preserve in vitro key features of the original tumour and preserve a certain degree of heterogeneity, elements that favour their use as models to predict clinical outcome. Thus, achieving robust and consistent organoid cultures is one of the most important immediate goals/tools to gain a thorough understanding of the processes underlying their generation, tumour formation and response to treatment. Ideally, cancer patients undergoing tumour resection should also have PDOs established from the different lesions detected, to help determine the next best course of therapy and facilitate the discovery of new therapies.
One of the major focuses of our laboratory is to implement patient derived organoids (PDO) as a tool to unravel fundamental mechanisms in cancer progression and resistance and better define treatment options. This type of approach does not come without own hurdles to overcome – reproducibility (in terms of yield, organoid size, shape, cellular composition and 3D architecture) is essential in order to understand the mechanisms that underlie organoid development in normal and pathological situations, and to use them as targets for manipulation or drug testing. Moreover, in the case of drug testing and regenerative medicine, it is crucial that, in addition to being reproducible, organoid systems are both scalable and safe.
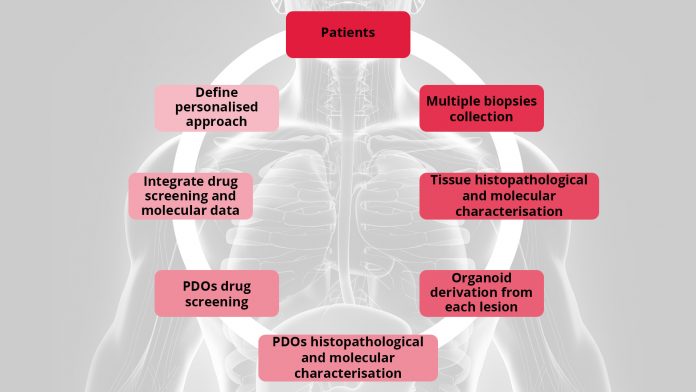
UroGenus PDO approach
Our laboratory has been successfully exploring different protocols options to better fit bladder and prostate organoids derivation, expansion, profiling and testing. To account for inter- and intra-patient heterogeneity we combine molecular characterisation of the tumour with functional evaluation of the PDOs chemo-sensitivity in order to tailor medical care to a patient’s individual genetic background.
Using organoids derived from three patient derived xenografts (PDXs), a treatment-naïve, soft tissue metastasis PDX, (PNPCa, established in our laboratory) and two metastatic PDXs (BM18, LAPC9), we have defined a 15 drug FDA-approved panel. Interestingly, multikinase inhibitors (ponatinib, sunitinib and sorafenib) were broadly effective on all PDXs organoids- and patient-derived organoids from advanced cases with acquired resistance to standard-of-care compounds.28 PDOs of BLCa (NMIBC and MIBC samples), grown in a defined medium retain tissue heterogeneity and when treated with a standard-of-care and the FDA-approved panel compound, they revealed differential drug sensitivity, responding to conventional chemotherapy and to targeted FDA-approved agents.
As a proof of principle, these results perfectly support our program that aims to collect and characterise biopsies from different lesions of prostate and bladder cancer patients. Correlation of the pre-clinical findings to clinical and molecular parameters is necessary in order to improve patient stratification and treatment regimens for personalised treatments.
Attributions
Marianna Kruithof-de Julio, PhD, PD
Head of the Urology Research Laboratory
Department of Urology & Department for BioMedical Research
Director Organoid CORE
Associate Member NCCR RNA and Disease
marianna.kruithofdejulio@dbmr.unibe.ch
Marta De Menna, PhD
Senior Scientist
Department for BioMedical Research
marta.demenna@dbmr.unibe.ch
Tweet
@MariannKrutihof
@DBMR_Unibe
References
- Rahib, L. et al. 2014. Projecting Cancer Incidence and Deaths to 2030: The Unexpected Burden of Thyroid, Liver, and Pancreas Cancers in the United States. Cancer Res 74, 2913–2921
- Siegel, R., Ma, J., and Zou, Z. et al. 2014. Cancer statistics, 2014. Ca Cancer J Clin 64, 9–29
- Bubendorf, L. et al. 2000. Metastatic patterns of prostate cancer: An autopsy study of 1,589 patients. Hum Pathol 31, 578–583
- Coleman, R. 2006. Clinical Features of Metastatic Bone Disease and Risk of Skeletal Morbidity. Clin Cancer Res 12, 6243s–6249s
- Witton, C., and Nielsen, K. 2008. Is PTEN loss associated with clinical outcome measures in human prostate cancer? Brit J Cancer 99, 1296–1301
- Yoshimoto, M. et al. 2008. Absence of TMPRSS2:ERG fusions and PTEN losses in prostate cancer is associated with a favorable outcome. Modern Pathol 21, 1451–1460
- Krohn, A. et al. 2012. Genomic Deletion of PTEN Is Associated with Progression and Early PSA Recurrence in ERG Fusion-Positive and Fusion-Negative Prostate Cancer. Am J Pathology 181, 401–412
- Burdelski, C. et al. 2015. Loss of SOX9 Expression Is Associated with PSA Recurrence in ERG-Positive and PTEN Deleted Prostate Cancers. Plos One 10, e0128525
- Hessels, D., and Schalken, J. 2013. Recurrent Gene Fusions in Prostate Cancer: Their Clinical Implications and Uses. Curr Urol Rep 14, 214–222
- Steurer, S. et al. 2014. TMPRSS2-ERG Fusions Are Strongly Linked to Young Patient Age in Low-grade Prostate Cancer. Eur Urol 66, 978–981
- Hoogland, A. et al. 2012. ERG immunohistochemistry is not predictive for PSA recurrence, local recurrence or overall survival after radical prostatectomy for prostate cancer. Modern Pathol 25, 471–479
- Minner, S. et al. 2011. ERG Status Is Unrelated to PSA Recurrence in Radically Operated Prostate Cancer in the Absence of Antihormonal Therapy. Clin Cancer Res 17, 5878–5888
- Pra, A. et al. 2013. TMPRSS2-ERG Status Is Not Prognostic Following Prostate Cancer Radiotherapy: Implications for Fusion Status and DSB Repair. Clin Cancer Res 19, 5202–5209
- Ploeg, M., Aben, K., and Kiemeney, L. 2009. The present and future burden of urinary bladder cancer in the world. World J Urol 27, 289–293
- Svatek, R. et al. 2014. The Economics of Bladder Cancer: Costs and Considerations of Caring for This Disease. Eur Urol 66, 253–262
- Babjuk, M. et al. 2012. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder, the 2011 update. Actas Urológicas Españolas Engl Ed 36, 389–402
- Sylvester, R., Meijden, A.P., andLamm, D. 2002. Intravesical bacillus calmette-guerin reduces the risk of progression in patients with superficial bladder cancer. j urology 168, 1964–1970
- Thalmann, G. et al. 2004. Primary t1g3 bladder cancer: organ preserving approach or immediate cystectomy? J urology 172, 70–75
- Avritscher, E. et al. Clinical model of lifetime cost of treating bladder cancer and associated complications. Urology 68, 549–553
- Pietzak, E. et al. 2017. Next-generation sequencing of nonmuscle invasive bladder cancer reveals potential biomarkers and rational therapeutic Targets. Eur Urol 72, 952–959
- Hedegaard, J. et al. 2-16. Comprehensive Transcriptional Analysis of Early-Stage Urothelial Carcinoma. Cancer Cell 30, 27–42
- Robertson, A. et al. 2018. Comprehensive Molecular Characterization of Muscle-Invasive Bladder Cancer. Cell 174, 1033
- Hafner, C. et al. 2001. Evidence for oligoclonality and spread by intraluminal seeding in multifocal urothelial carcinomas of the upper and lower urinary tract. Oncogene 20, 4910–4915
- Hafner, C., Knuechel, R., and Stoehr, R. et al. 2002. Clonality of multifocal urothelial carcinomas: 10 years of molecular genetic studies. Int J Cancer 101, 1–6
- Yoon, D. et al. 2001. Genetic mapping and DNA sequence-based analysis of deleted regions on chromosome 16 involved in progression of bladder cancer from occult preneoplastic conditions to invasive disease. Oncogene 20, 5005–5014
- Strandgaard, T. et al. 2019. Mutational analysis of field cancerization in bladder cancer. Biorxiv 536466.Available at: doi:10.1101/536466
- Höglund, M. and Kirkali, Z. 2007. On the Origin of Syn- and Metachronous Urothelial Carcinomas. Eur Urol 51, 1185–1193
- Karkampouna, S. et al. 2020. Patient-derived xenografts and organoids model therapy response in prostate cancer. Biorxiv 2020.03.17.994350.Available at: doi:10.1101/2020.03.17.994350