DFG Research Consortium, ‘ExCarBon’, explores articular cartilage and subchondral bone degeneration and regeneration in osteoarthritis.
Osteoarthritis (OA), a whole-joint disease in which all components of the joint are affected, is a leading cause of disability in older adults. OA emanates from the dysfunction of the whole joint, especially articular cartilage (AC), synovium (SY) and subchondral bone (SB), three tissues with intimate mechanical and biological interactions. During the evolution of OA pathology, the compositions, functional properties, and structures of these tissues undergo marked alterations.
Although pathological processes might selectively target a single joint tissue, ultimately all of the components of the osteochondral unit will be affected because of their intimate association, and thus the biological and physical crosstalk among them is of great importance. Evidence from studies in human and animal models also suggests that OA is associated with chronic low-grade systemic and local inflammation. Inflammatory mediators include key components of the innate immune system (complement system, macrophages and mast cells) and cytokines, adipokines, prostaglandins and neuropeptides.1,2
With an ageing and increasingly obese population, OA is becoming even more prevalent with knee OA accounting for approximately 85% of OA worldwide. OA accounted for 3.9% of years lived with disability worldwide in 2015, and by 2020 it is expected to be the fourth leading cause of years lived with disability globally.3 A large proportion of patients experience pain as the most disabling symptom, with pain sensitisations by means of nociceptive, inflammatory and neuropathic pain mechanisms arising from structural changes in the joint innervation or from nerve changes in the peripheral nervous system or spinal cord.4
Therefore, the development of targeted therapies against the osteoarthritic processes in cartilage, synovium or bone requires an understanding of the state of these joint tissues at the time of the intervention. Importantly, these interventions will not be successful unless they are applied at the early stages of the disease before considerable structural and functional alterations occur in the osteochondral unit.5,6 A number of stratifications have been proposed on the basis of specific pathological processes to classify different mechanistic subgroups, which include an increased inflammatory component, mechanical overload, metabolic alterations, and cell senescence.7
Although OA can be initiated by multiple factors at multiple sites, mechanical overloading is still the key feature of OA pathogenesis. Joint tissues are sensitive to physical stimuli and OA may result from excessively aberrant or physiologically normal mechanical stresses on initially healthy or pathologically-impaired articular cartilage, bone and ligaments, respectively.8 There is an obvious need to elucidate the mechano-pathophysiology of OA and define the biomechanical and biochemical mechanisms involved between the articular cartilage and subchondral bone in order to identify novel targets and develop therapies to improve the quality of life in OA patients.
Mission and objectives
ExCarBon wants to dissect molecular pathways and cellular responses pivotal for articular cartilage and subchondral bone maintenance and destruction during OA. This can be done by using imaging and nanomechanical modalities to assess structure-to-function relationships. Inflammatory cell responses in joint tissues and its relationship with onset and progression of OA need to be characterised. It is crucial to analyse the effect of mechanical stimuli and the physical microenvironment on cell-based regeneration processes.
Our overall research concept connects cartilage/synovial/bone pathophysiology to regenerative osteo-chondrogenesis. We expect our integrated research activities to result in a comprehensive understanding of joint tissue pathophysiology and to identify and verify novel targets for diagnosis and therapeutic intervention in OA. This is expected to result in the development of novel strategies to halt OA progression and in improved therapies for the treatment of osteochondral lesions and synovial inflammation.
During the previous work of ExCarBon, several aspects of OA pathology have yielded important insights into the progression of joint tissue degeneration in the frame of our clustered projects. Here, we further tailored our focus for the next years identifying six target areas, which require extensive collaboration and shared technologies among the consortium.
The role of extracellular matrix and signalling in osteoarthritis
OA development is associated with a sequence of pathological changes of the articular cartilage extracellular matrix (ECM). We have shown that interfering with heparin sulphate biosynthesis or integrin-ECM interactions has a clear impact on the structure and/or composition of the cartilage matrix, and on the organisation and function of the chondrocyte cytoskeleton. Our emerging concept shows those changes have a pivotal role in the initiation and onset of OA by altering cartilage biomechanics and disrupting the ECM-cytoskeleton connection.
Mechanotransduction in joint tissues
All types of cells in the joint are continuously exposed to mechanical forces, including external and reactive forces caused by the mechanical properties of the extracellular environment. Cells can sense and respond to mechanical forces, which play critical roles in various cellular activities, including motility, morphological changes, proliferation, differentiation and polarity formation as well as tissue morphogenesis, OA pathogenesis and AC repair. Previously we have revealed the importance of mechanotransduction in chondrocyte and mesenchymal stem cell (MSC) physiology as well as for subchondral bone microarchitecture.
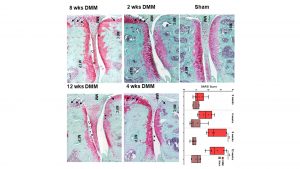
Calcified cartilage and bone changes in osteoarthritis
Alterations in calcified cartilage, subchondral bone, osteochondral junctions and other bony structures are frequently associated with OA pathogenesis. Histological and high-resolution nanoCT-aided analyses have demonstrated modification of the calcified tissues in almost all genetically modified, surgical OA and repair animal models of ExCarBon, further corroborating the importance of SB in OA pathogenesis. We will continue evaluating structural and molecular changes in bone and its mutual interaction with cartilage biomechanics in the next years.
Peripheral nervous system in OA and repair
We have previously shown that changes in sensory and sympathetic neurotransmitter profiles and the absence of their neurotransmitters contribute to degenerative alterations in joint tissues and alter MSC-mediated repair processes in vitro. We will further study the role of the peripheral nervous system in OA pathophysiology using mechanical instability models, animal models under different stress conditions and will correlate the sympathetic tone with OA symptoms in human patients.
Priming stem cells for AC repair
Articular cartilage has minimal ability for self-repair, resulting in progressive tissue loss and dysfunction following cartilage injuries and during spontaneous OA. Stem cells have enormous potential to contribute to novel treatment strategies for the repair of chondral or osteochondral defects. Previous studies within ExCarBon have identified several promising strategies for priming MSC for more efficient chondrogenic differentiation. We will continue to discover the molecular mechanisms underlying the effect of chondrogenic stimuli at genetic, epigenetic, transcriptome and protein levels. Furthermore, we will extend the priming process for induced pluripotent stem cells (iPSC) to enhance their differentiation efficacy.
Inflammation in OA and AC repair
In contrast to the simplified view that OA is merely a ‘wear and tear’ disease, it is now clear that pro-inflammatory cytokines and cellular mediators play an important role in the onset and perpetuation of this disease. Synovitis is also associated frequently with joint injury and maybe a prognostic indicator of the rate of development of posttraumatic OA. As demonstrated, inflammatory changes are part of the OA complex phenotype. Over the upcoming years, we propose to analyse the influence of synovitis and inflammation on OA pathology. We expect to identify unique cellular targets preventing OA-inducing inflammation and to test therapeutic utilities to deplete specific inflammatory cells.
Expected benefits and impact
It is the vision of this consortium to contribute to the understanding of articular cartilage, synovial tissue and subchondral bone homeostasis and biomechanics, degeneration and regeneration with the ultimate aim of defining reliable targets for therapy of chondral/osteochondral defects. This will promote effective joint repair and provide alternatives for joint replacement or the reliance on a wheelchair of end-stage OA patients. The successful treatment and prevention of OA present a significant challenge due to an insufficient knowledge about functional interactions among joint tissues (e.g. cartilage, synovium and subchondral bone) and the cumulative effects of risk factors such as age, inflammation, metabolic and neuronal dysfunction, genetic predisposition and inappropriate or aberrant mechanical stress. Furthermore, it has become increasingly evident that a deep understanding of mechanobiology and mechanotransduction is not only crucial for joint development, homeostasis and pathology, but is fundamental to develop successful individualised, patient adapted treatment strategies.
References
1 Scanzello, C.R., and Loeser. R.F. 2015. ditorial: inflammatory activity in symptomatic knee osteoarthritis: not all inflammation is local, Arthritis & rheumatology. 67(11) (2015) 2797-800
2 Robinson, W.H., Lepis, C.M, and Wang, Q. et al. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nature reviews:Rheumatology 12(10) (2016) 580-92
3 Hunter, D.J., Schofield, D., and Callander, E. 2014. The individual and socioeconomic impact of osteoarthritis. Nature reviews: Rheumatology 10(7) (2014) 437-41
4 Fu, A., Robbins, S.R., and McDougall, J.J. 2018. Osteoarthritis: the genesis of pain. Rheumatology 57(suppl_4) (2018) iv43-iv50
5 Loeser, R.F., Goldring, S.R., and Scanzello, C.R. et al. 2012. Osteoarthritis: a disease of the joint as an organ. Arthritis and rheumatism 64(6) (2012) 1697-707
6 Goldring, S.R., and Goldring, M.B. 2016. Changes in the osteochondral unit during osteoarthritis: structure, function and cartilage-bone crosstalk. Nature reviews:Rheumatology 12(11) (2016) 632-644
7 Hunter, D.J., and Bierma-Zeinstra, S. 2019. Osteoarthritis. Lancet 393(10182) (2019) 1745-1759
8 Guilak, F. 2011. Biomechanical factors in osteoarthritis. Best.Pract.Res.Clin.Rheumatol. 25(6) (2011) 815-823
Susanne Grässel & Attila Aszodi
Spokesperson & Co-spokesperson
ExCarBon
+49 9419435065
susanne.graessel@klinik.uni-regensburg.de
www.excarbon.com/
Please note, this article will also appear in the first edition of our new quarterly publication. Subscribe to our updates for free here.







