Safety testing in battery calorimeters paves the way for improved understanding and prevention of thermal runaway.
Established in 2011, the Calorimeter Center at the Karlsruhe Institute of Technology’s (KIT) Institute for Applied Materials – Applied Materials Physics, now operates Europe’s largest battery calorimeter laboratory. It provides six Accelerating Rate Calorimeters (ARCs) of different sizes – from coin to large pouch or prismatic automotive format, which allow the evaluation of thermodynamic, thermal and safety data for Lithium-ion and post-Lithium batteries on material, cell, and pack level for both normal and abuse conditions (thermal, electrical, mechanical).
Safety first
Safety comes first – this is the mission of the centre’s head, Dr Carlos Ziebert. It is clear that safety issues have a major influence on consumers’ willingness to adopt battery systems. A holistic safety assessment is a prerequisite for upscaling and market acceptance of battery technologies, because an uncontrollable increase in temperature of the entire system (so-called ‘thermal runaway’) can cause ignition or even explosion of the cell that leads to negative public attention or even rejection.
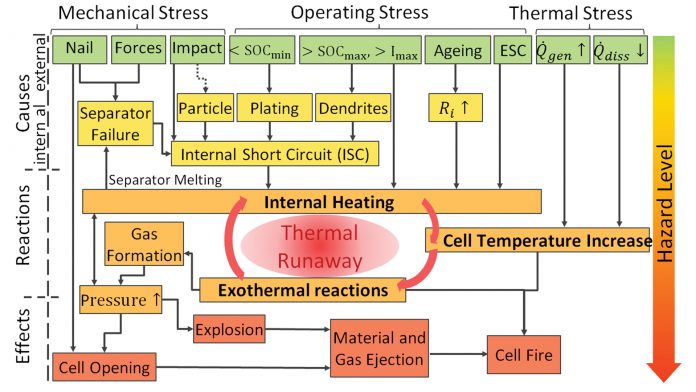
The causes and effects of thermal runaway can be very diverse and complex (see Figure 1). Either internal or external mechanical, operating, or thermal stresses lead to an internal heating of the cell that initiates different exothermal reactions, followed by a further temperature and pressure increase. The final effects can be empirically classified by the Hazard Level (1-7). Cell designs, component integrity, manufacturing, and ageing processes all have critical influence on the safety of Li-ion batteries.
Battery calorimetry benefits
Calorimetry – or the process of measuring heat data during chemical reactions – allows the collection of quantitative data required for optimum battery performance and safety. Sophisticated battery calorimetry combined with thermography allows finding new and quantitative correlations between different critical safety and thermally related parameters. This is very important because you need to know how many Watts a cell will produce under every condition in order to adapt the battery and thermal management systems. The temperature, heat, and internal pressure evolution can be studied, while operating cells under conditions of normal use, abuse, or accidents. Such abuse tests without a calorimeter have two main disadvantages:
- The maximum safe temperature would be underestimated;
- The consequences would be understated in terms of severity and speed;
- Moreover, a test in the calorimeter is much more sensitive than a hotbox test and reveals the entire process of the thermal runaway with the different stages of exothermic reactions. These data are essential for battery and thermal management as well as safety system design. Combined with multiscale electrochemical-thermal modelling they provide a powerful tool for thermal runaway prevention and ageing prediction.
Safety testing
A typical ARC chamber has heaters and thermocouples located in the lid, bottom, and side wall, which adjust the required ambient conditions. ARCs provide thermal stability data at the materials level, and allows safety tests to be performed at the cell and pack level by applying:
1) Electrical abuse: external/internal
short circuit test, overcharge test, overdischarge test. In the ARC the temperature increases by applying an external short circuit or during an internal short circuit, which might be caused, for example, by a production fault, can be measured. The cell can be overcharged or over discharged leading to different failure modes.
2) Mechanical abuse: nail penetration test. In the ARCs, a mechanical system allows to push a nail into the cell. This provides not only a pass/fail type test to qualify cells, but also quantitative data.
3) Thermal abuse: Heat-Wait-Seek test, ramp heating test, thermal propagation test.
The Heat-Wait-Seek (HWS) test starts in the Heat Mode by heating up the cell in small temperature steps (typically 5K), as shown in the flowchart in Figure 2. At the end of each step, the Wait Mode is activated to reach thermal equilibrium. Then, the system enters Seek Mode, which seeks the temperature rate and ends with two possible modes: Exotherm Mode or Heat Mode. If the measured temperature rate is larger than the onset sensitivity (typically 0.02K/min), which means that self-heating of the cell is detected, the system goes into Exotherm Mode. In this quasi- adiabatic mode, the heaters in the calorimeter chamber immediately follow any change of the cell temperature, preventing the heat transfer to the chamber. Thus, it is increasingly heating up until a thermal runaway occurs or the chemicals are completely consumed by the exothermic reactions. On the other hand, if the temperature rate is smaller, the system goes back into Heat Mode. If the temperature exceeds the end temperature value, the ARC enters Cool Mode, switches off the heaters and starts to cool down by introducing pressurised air to the chamber.
For the Ramp Heating test, which mimics a Hot Box test, the cells are heated up at a constant rate instead of a stepwise heating of the cells as in the HWS test. Moreover, the calorimeters allow studying the thermal runaway propagation using different initiation methods in order to develop and qualify suitable countermeasures, such as heat protection barriers.
In addition, new methods for the measurement of internal cell pressures for early prediction of thermal runaway of LIB have been established on 18650 cells. A pressure line connected to a pressure transducer was directly inserted into the cell and the pressure was recorded during a HWS test, as can be seen in Figure 2. This plot clearly shows that a pressure increase occurs much earlier than self-heating, and that the cell goes into thermal runaway even if the safety vent opens and releases gases leading to a pressure drop. Thus, the measurement of the internal pressure could be used for the early prediction of processes leading to thermal runaway. This method has been transferred from cylindrical cells to pouch cells and prismatic automotive cells.
As a result of the different tests, quantitative data and system relevant data for temperature, heat and pressure development of the cells are provided as:
- A fast feedback for the cell developers;
- Essential data for modelling and simulation; and
- Required data for the design and adaptation of battery management systems.
There are still numerous challenges to address and we hope that our Calorimeter Center will help the European industry to make further progress in the battery field, which is urgently needed to reach a low-carbon future, to foster European leadership, and to create new jobs, which are the main objectives of the European Battery Alliance.
Dr Carlos Ziebert
Institute of Applied Materials Physics
Karlsruhe Institute of Technology
+49 721 608-22919
carlos.ziebert@kit.edu
www.iam.kit.edu/awp/english/